Do 3am wakeups age you? Low-quality sleep = “older than expected” DNA methylation ages in midlife and beyond.
Most conversations about sleep and aging focus on how we feel. Epigenetic clocks measure something different: how fast our cells are aging.
The data from these clocks now reframe sleep from a quality-of-life issue to a longevity variable, namely:
Chronic sleep disruption accelerates biological aging.
Short sleep, frequent 3 AM awakenings, and fragmented sleep are now consistently associated with faster biological aging on multiple molecular markers.
Specifically, biological age markers such as DNA methylation–based epigenetic clocks estimate how fast an individual is aging compared to chronological years.
And, across cohorts in midlife and older age, individuals with sleep disruptions often show older epigenetic ages & shorter telomeres than peers with better sleep.
The important counterpoint is that sleep is a modifiable factor.
So, the natural question is: does improving sleep quality slow or partially reverse these aging markers?
The sections that follow review the human data that speak to that question, with a focus on what they imply for individuals in midlife and older age.
Below, we discuss the latest evidence:
- linking poor sleep to accelerated epigenetic aging &
- emerging data on whether better sleep and sleep-focused programs can slow these markers in midlife and beyond.
Section 1. Sleep Issues & Epigenetic Age Acceleration, Longevity
Multiple studies now link sleep problems to epigenetic age acceleration (EAA) – a state where one’s DNA methylation age exceeds their chronological age.
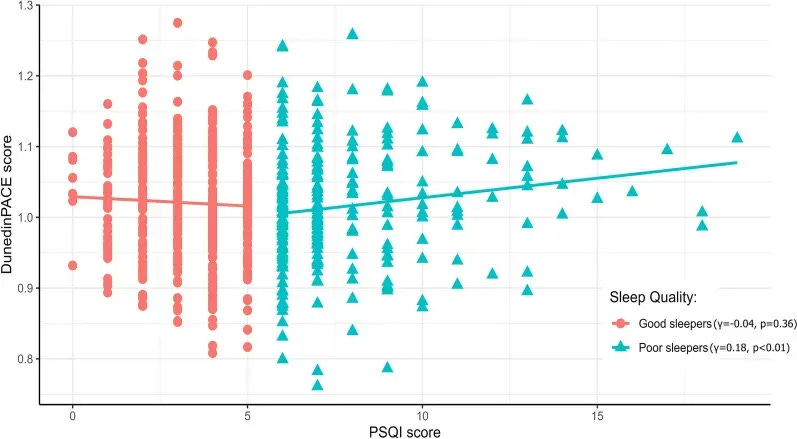
2024 cohort of ~700 middle-aged adults: poor sleep quality → faster epigenetic aging
A 2024 study in ~700 middle-aged adults showed that sleep fragmentation and quality, not just duration, are important.
Worsening sleep quality, as measured by the Pittsburgh Sleep Quality Index (PSQI—a composite of subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, daytime dysfunction), was significantly linked to faster biological aging.
Among participants classified as poor sleepers, each increment of higher PSQI score (worse sleep) was associated with:
- a faster aging pace on the DunedinPACE clock, and
- higher GrimAge acceleration,
whereas among good sleepers, PSQI scores showed little association with these clocks.
2017 UCLA Study of > 2,000 older adults: insomnia burden → older epigenetic age
A large cohort of 2,078 older women in the United States (mean age ~64 years) found that individuals with more frequent insomnia symptoms had older epigenetic ages than their peers with no insomnia symptoms.
Researchers assessed five insomnia dimensions:
- trouble falling asleep,
- waking frequently during the night,
- difficulty returning to sleep,
- waking earlier than planned, and
- restless or non-restorative sleep.
Several patterns emerged:
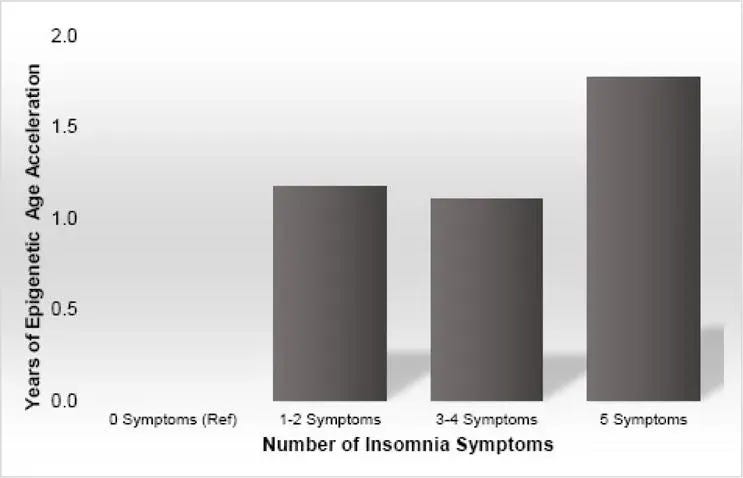
- Epigenetic age acceleration (EEAA) increased with the number of insomnia symptoms. Individuals with no symptoms had the youngest epigenetic ages, followed by those with 1–2 symptoms, then 3–4 symptoms, and those with 5 symptoms, who showed the oldest epigenetic ages.
- Women reporting any insomnia symptoms at least 1–2 times per week had older epigenetic ages compared with women reporting no symptoms.
When individual symptoms were examined, one stood out: those who reported waking during the night had higher epigenetic age acceleration (EEAA) compared with those who did not wake at night. The other individual symptoms, on their own, did not independently predict epigenetic age acceleration after adjustment.
These human studies—align with mechanistic biology and point in the same direction: habitual sleep disruption is associated with faster aging at a molecular level.
What these epigenetic aging findings imply
These human cohorts, converge on the same theme: habitual sleep disruption is associated with molecular signatures of faster aging. Poor sleep quality, frequent night awakenings, and composite insomnia burden all track with epigenetic clocks that predict higher mortality risk and a faster pace of aging.
Mechanistic work provides a link between these patterns and downstream biology. Chronic sleep disruption is associated with DNA methylation changes, histone and chromatin modifications related to stress signaling, and shorter telomeres that reflect reduced cellular replicative capacity.
Sleep OS Hormones is now available as a 60-day self-guided program with dedicated systems for estrogen, progesterone, and testosterone, or bundled together for a more complete approach.
This sets the stage for the next question: if poor sleep is linked to accelerated biological aging, can improving sleep quality and continuity slow, or partially reverse, these aging markers in midlife and older adults?
Section 2. Does Improving Sleep Slow Biological Aging?
Encouragingly, the latest evidence indicates that improving sleep can slow or even reverse certain aging biomarkers.
2025 cohort of 3,566 adults: better sleep → lower mortality risk & younger epigenetic age across 4 clocks
Among current human data, one of the most powerful pieces of evidence that sleep is a modifiable lever on biological aging comes from a 2025 analysis of 3,566 middle-aged and older adults (average age ~65 years).
Researchers built a composite “healthy sleep pattern” score from four modifiable sleep metrics:
- sleeping long enough at night
- maintaining good subjective sleep quality
- keeping a regular sleep–wake schedule
- limiting daytime napping
Participants with better sleep had younger DNA methylation ages across multiple clocks and a lower DNA methylation–derived mortality risk score.
- Each 1-point higher sleep score was associated with lower DNAm age acceleration across all four clocks: PhenoAgeAccel, GrimAgeAccel, DunedinPACE, DNAm MS.
- Slower DunedinPACE mediated ~6% of the association between healthier sleep patterns and lower all-cause mortality risk
Importantly, these models were adjusted for age, sex, education, smoking, alcohol use, physical activity, and body mass index.
That means the way you sleep is one of the levers you can improve to influence how fast your biological clocks are moving, even if the rest of your lifestyle already looks healthy.
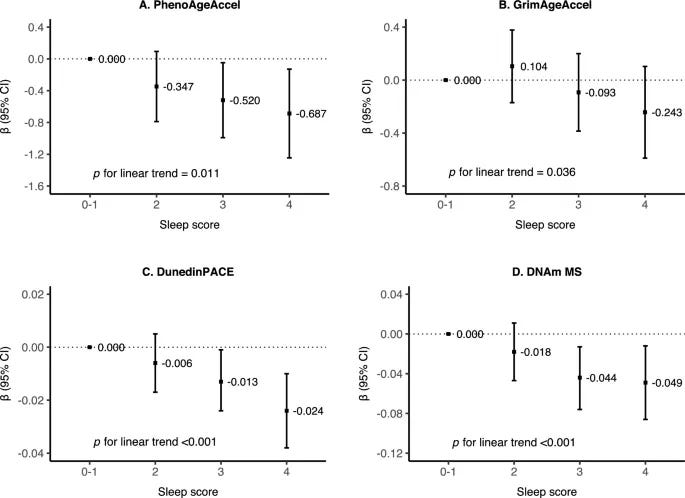
2023 controlled trial: insomnia remission in older adults → slowed biological aging
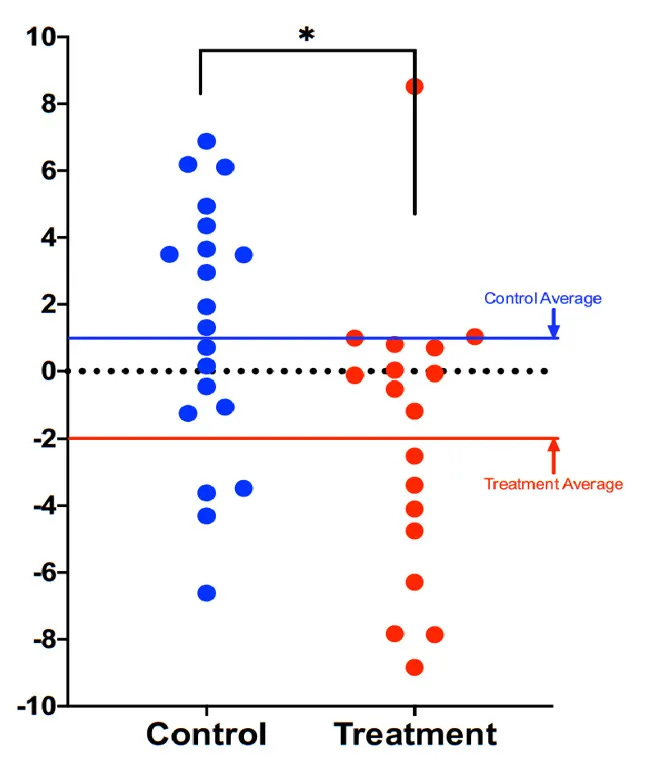
A 2023 randomized control trial (RCT) in 94 older adults (age 60+) with chronic insomnia found that successfully resolving insomnia slowed the biological pace of aging. Importantly, the comparison was not against “no treatment,” but against a sleep education therapy (SET) group focused on sleep hygiene.
Key findings:
- Compared with the sleep hygiene group, the insomnia remission group showed a significant treatment-by-time interaction on DunedinPACE (p = 0.003): the sleep improvement group showed a decline in the pace of biological aging, while the sleep hygiene group showed an increase in the pace of biological aging.
- Adults whose insomnia resolved also saw reduction in a cellular aging marker (p16^INK4a) measured in immune cells (PBMCs), while those without sustained remission showed rising levels, suggesting a buildup of senescent cells.
Taken together, this RCT shows evidence that improving sleep can slow the biological pace of aging and reduce a senescence marker in older adults.
Even in young adults: better sleep → younger epigenetic age
In early adulthood, short and irregular sleep already appears on the radar of epigenetic clocks. In a 9-week study of young adults, daily sleep logs and blood samples at the beginning and end of the study period were used to measure epigenetic vs chronological age.
Key findings:
- Participants with longer and more regular sleep showed a reduction in epigenetic minus chronological age difference.
- Those with shorter and more irregular sleep showed an increase in epigenetic age relative to chronological age (mean change about +3 years).
This study reinforces the idea that sleep quality and epigenetic aging can track together over just weeks, not only across decades.
Lifestyle program with sleep optimization → younger epigenetic age
A 2021 randomized trial in 43 men aged 50–72 tested an 8-week lifestyle program that included explicit sleep optimization alongside diet, exercise. Participants were instructed to obtain at least 7 hours of sleep per night as part of the study.
Key findings:
- The intervention group showed a 3.23-year decrease in Horvath DNAmAge relative to controls.
- Improvements in cardiometabolic markers in the intervention group (for example, higher serum 5-methyltetrahydrofolate and lower triglycerides).
The results suggest that improvements in lifestyle, including sufficient sleep, can rejuvenate epigenetic profiles.
Sleep is a modifiable factor on the biological aging trajectory
In summary, a convergence of evidence from epidemiological cohorts, clinical trials, and mechanistic studies indicates that sleep is a powerful lever for biological aging.
- Better sleep patterns (adequate duration, continuity, and regular timing) are associated with slower epigenetic clocks & lower DNA-based mortality risk in midlife and older adults.
- Partial improvements matter: addressing even one or two dimensions of poor sleep is associated with better epigenetic outcomes than addressing none.
- Middle-of-the-night awakenings, specifically, emerged as an independent predictor of epigenetic age acceleration
- When insomnia is actively resolved in later life, both the biological pace of aging (DunedinPACE) and senescence markers (p16^INK4a) move in a favorable direction
- Even in young adults, small, real-world changes in sleep duration and regularity appear to nudge epigenetic age either closer to or further from chronological age over only a few weeks.
Mechanistically, these shifts likely reflect changes in oxidative stress handling, DNA repair activity, and inflammatory signaling that accompany improvements in sleep continuity and timing.
In practical terms, the current evidence supports a clear message: sleep is not only a marker of how fast you are aging; it is one of the actionable levers that you can adjust to slow that trajectory.
Section 3. Most factors that influence biological aging—genetics, early life exposures, decades of accumulated wear—are not ones you can revisit.
Sleep is different.
For adults in midlife and beyond, this reframes sleep from a background habit to a primary lever for long-term vitality. Addressing sleep disruption is not just about feeling better the next morning; it is a way to help preserve genomic regulation, maintain tissue resilience, and support healthier aging over the coming decades.
Because sleep remains modifiable at virtually any age it is an actionable target for anyone aiming to slow their biological aging trajectory & extend their years of high function.
In my own work with adults their 50s and 60s+, I have seen sleep quality return to levels they had not experienced in 15-20 years once they addressed the right hormonal levers. It is possible.
How you sleep this year is shaping how fast (or slow) you age into the next ones.
Warmly,
—Kat
P.S. If your sleep issues began after 40, your nervous system is doing the best it can around a new hormonal baseline. Sleep OS: Hormones helps you work on that baseline directly—without sleep medications, without more supplements, and without costly hormone workups—so you can reestablish full-night sleep and reduce the pressure sleep loss puts on brain aging and dementia risk.
👉 You can learn more about my most popular program, the Trio Hormone Sleep Recovery Course, here:
👉 Or, explore the foundational Sleep & Stress Single Hormone Sleep Recovery Course, here:
Here’s what that shift can look like:
A recent Sleep OS member who worked through the Hormone Frameworks described it this way: ‘I still get up once to use the bathroom, but I fall back asleep in seconds and I’m actually satisfied with my sleep again.’
References
- Diao, T., Liu, K., Zhou, L. et al. Sleep patterns and DNA methylation age acceleration in middle-aged and older Chinese adults. Clin Epigenet 17, 87 (2025).
- Rivero-Segura NA, Cuartas JDR, Garcia-delaTorre P, Sanchez-Garcia S, Ramirez-Aldana R, Gomez-Verjan JC. Insomnia accelerates the epigenetic clocks in older adults. Geroscience. 2025 Dec;47(6):6777-6788.
- Zhao, W., Yu, S., Xu, Y. et al. Sleep traits causally affect epigenetic age acceleration: a Mendelian randomization study. Sci Rep 15, 7439 (2025).
- Lee HS, Kim B, Park T. The association between sleep quality and accelerated epigenetic aging with metabolic syndrome in Korean adults. Clin Epigenetics. 2024 Jul 16;16(1):92.
- Chen, M., Wang, Z., Xu, H. et al. Association between modifiable lifestyle factors and telomere length: a univariable and multivariable Mendelian randomization study. J Transl Med 22, 160 (2024).
- Judith Carroll, COGNITIVE BEHAVIORAL TREATMENT OF INSOMNIA SLOWS THE PACE OF BIOLOGICAL AGING: RESULTS FROM AN RCT IN OLDER ADULTS, Innovation in Aging, Volume 7, Issue Supplement_1, December 2023, Pages 205–206
- Fitzgerald KN, Hodges R, Hanes D, Stack E, Cheishvili D, Szyf M, Henkel J, Twedt MW, Giannopoulou D, Herdell J, Logan S, Bradley R. Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial. Aging (Albany NY). 2021 Apr 12;13(7):9419-9432.
- Carskadon, M.A., Chappell, K.R., Barker, D.H. et al. A pilot prospective study of sleep patterns and DNA methylation-characterized epigenetic aging in young adults. BMC Res Notes 12, 583 (2019).
- Carroll JE, Irwin MR, Levine M, Seeman TE, Absher D, Assimes T, Horvath S. Epigenetic Aging and Immune Senescence in Women With Insomnia Symptoms: Findings From the Women’s Health Initiative Study. Biol Psychiatry. 2017 Jan 15;81(2):136-144.
- Mean difference in extrinsic epigenetic age acceleration by number of insomnia symptoms. (Carroll JE, Irwin MR, Levine M, Seeman TE, Absher D, Assimes T, Horvath S. Epigenetic Aging and Immune Senescence in Women With Insomnia Symptoms: Findings From the Women’s Health Initiative Study. Biol Psychiatry. 2017 Jan 15;81(2):136-144.
- Zhang X, Wang Y, Zhao R, Hu X, Zhang B, Lv X, Guo Z, Zhang Z, Yuan J, Chu X, Wang F, Li G, Geng X, Liu Y, Sui L, Wang F. Folic Acid Supplementation Suppresses Sleep Deprivation-Induced Telomere Dysfunction and Senescence-Associated Secretory Phenotype (SASP). Oxid Med Cell Longev. 2019 Dec 14;2019:4569614.
- Carroll JE, Cole SW, Seeman TE, Breen EC, Witarama T, Arevalo JMG, Ma J, Irwin MR. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun. 2016 Jan;51:223-229.