Chronic inflammation is one of the most powerful—yet often subtle—drivers & signatures of aging, brain decline, and disease progression. It’s central to the growing field studying inflammation and aging.
It doesn’t just raise your risk for heart disease or diabetes. It can also accelerate brain aging, disrupt metabolic health, and quietly push you toward neurodegeneration.
That’s why measuring inflammatory markers is one of the smartest longevity moves you can make.
Get Weekly Insights to Personalize Your Own Longevity Roadmap
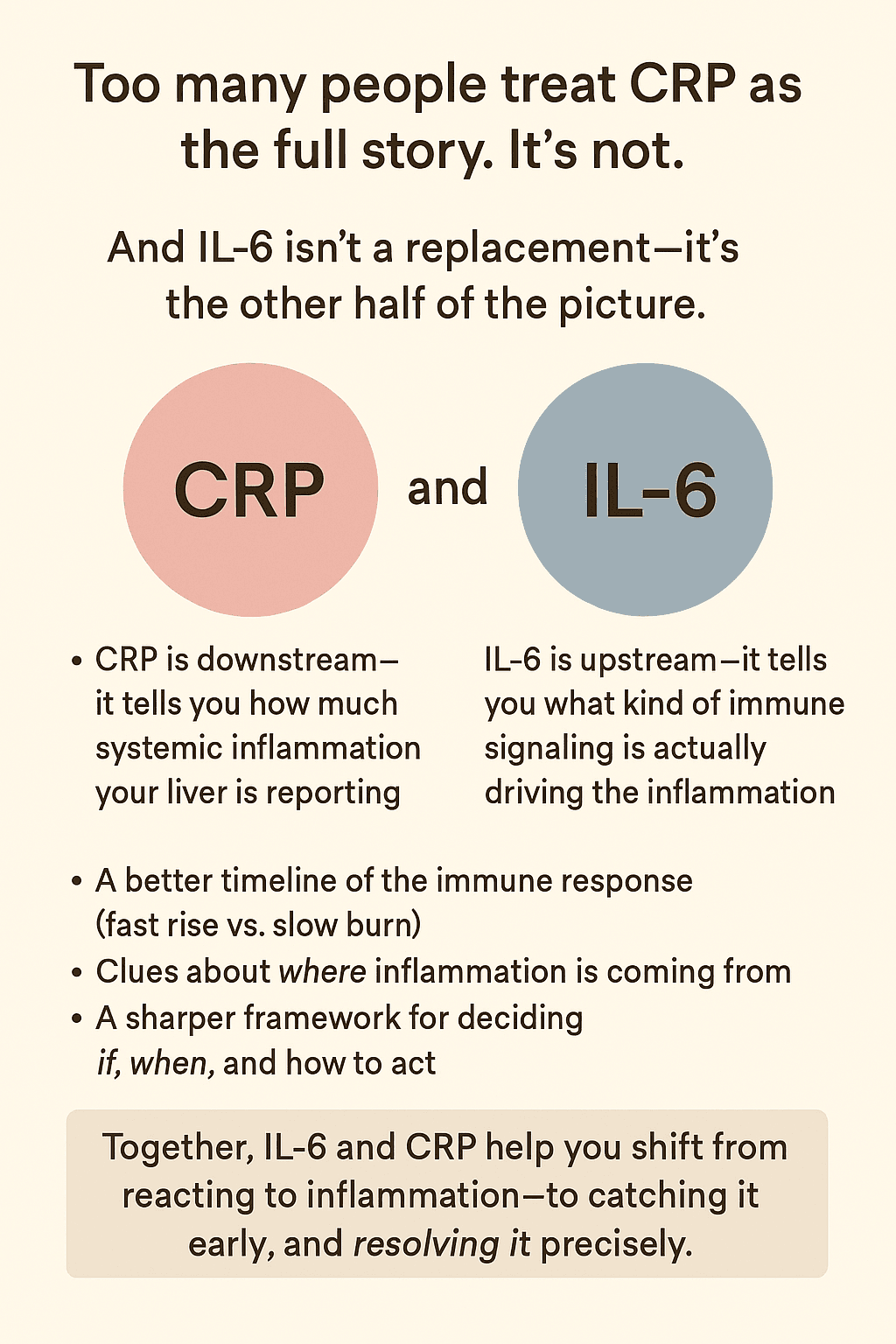
Most people have heard of hs-CRP, the high-sensitivity C-reactive protein test. It’s a valuable tool—especially for detecting long-term, low-grade inflammation.
But it doesn’t tell the full story.
To understand the mechanisms of inflammation and aging and its impact on longevity, you need to go upstream—to IL-6.
Table of Contents
1. Why Understanding IL-6 and Inflammation Is Key to Longevity Optimization
What is interleukin 6 (IL-6)?
It’s a cytokine deeply tied to the earliest immune signals that drive aging. Measuring it with an IL-6 blood test provides a more sensitive, earlier view of hidden inflammation
In fact, studies show that patients with elevated IL-6 blood test results face a higher risk of cardiovascular events—even when their hs-CRP levels are low or normal (PMC).
In this article, we’ll break down why IL-6 matters so much for brain health, aging, and disease prevention—and why we recommend testing both hs-IL-6 and hs-CRP if you’re serious about optimizing for longevity.
2. 🧠Interleukin-6 and Longevity: An Actionable Marker
2A. IL-6, Inflammation, and Cognitive Decline
We often think of inflammation as something that happens in our joints or gut—but when it shows up in the brain, the long-term consequences can be devastating. That’s where IL-6 comes in: a key driver of inflammation in the brain and an early marker of cognitive risk.
This cytokine—central to the relationship between IL-6 and inflammation— has emerged as one of the most predictive inflammatory markers for age-related cognitive decline.
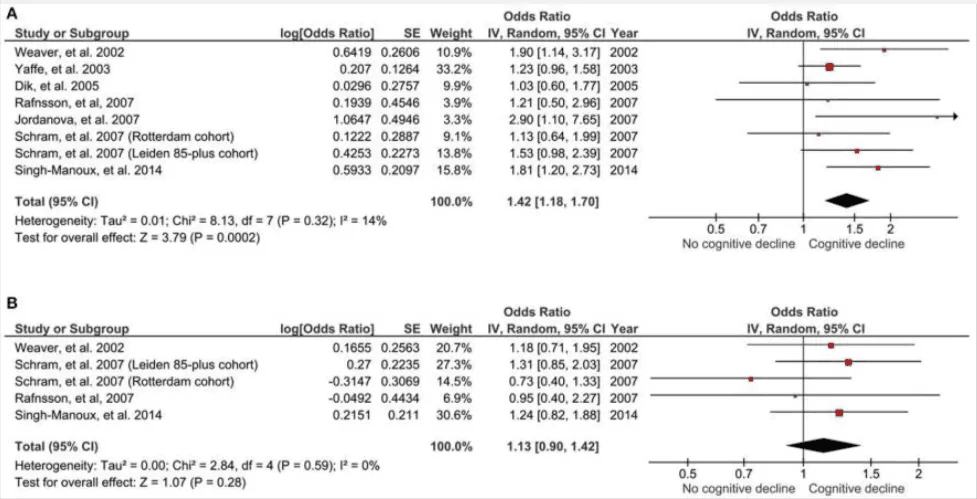
In a large-scale meta-analysis involving over 15,000 participants, researchers found that individuals with elevated IL-6 levels were 42% more likely to experience global cognitive decline over a 2–7-year period than those with lower levels. This relationship held up even after adjusting for baseline cognitive scores and comorbidities, highlighting IL-6 as a stand-alone signal—not just a side effect of poor health.
There’s more.
Higher IL-6 levels aren’t just linked to slower thinking or memory lapses. In another study of over 50,000 participants, it was found that higher IL-6 levels are associated with:
- a 23-25% increased risk of all-cause dementia (PubMed).
- reduced brain volume,
- poorer cognitive performance.
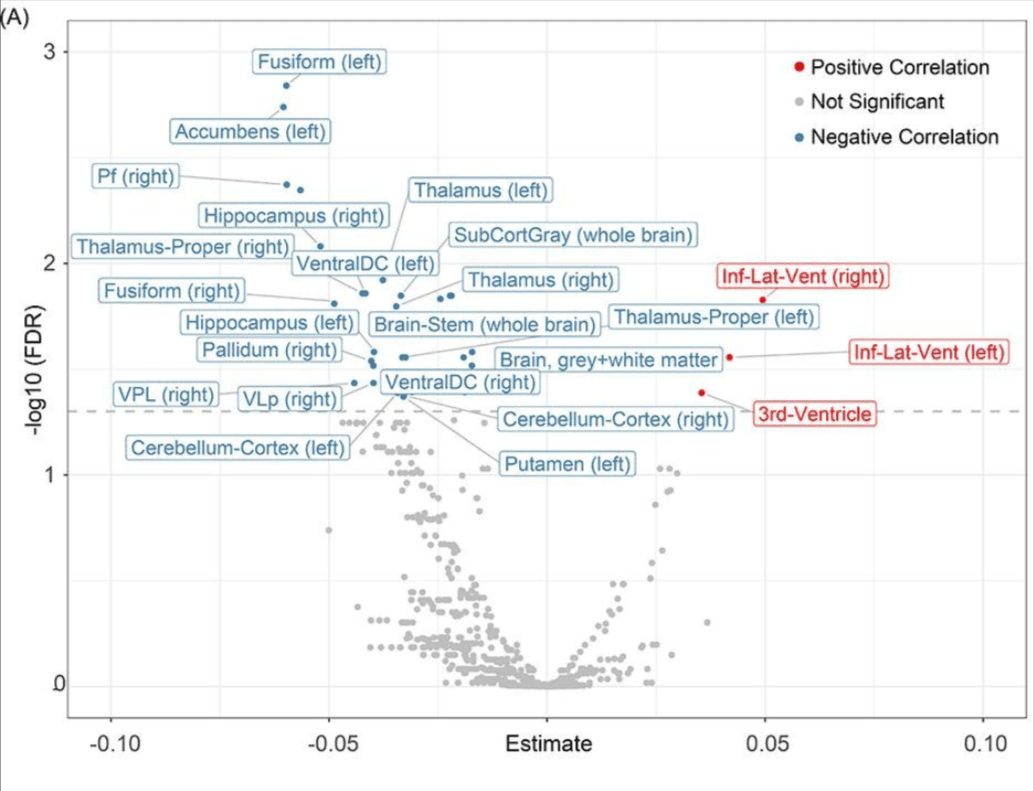
In other words, IL-6 doesn’t just track with cognitive symptoms—it seems to precede and even help drive them.
And unlike hs-CRP, which often lags behind the inflammatory process, IL-6 offers a faster and more direct look at what’s happening in the brain’s immune environment—especially when measured with high-sensitivity assays.
For those looking to preserve cognition over the long haul, hs-IL-6 testing may be one of the most underrated early warning systems we have.
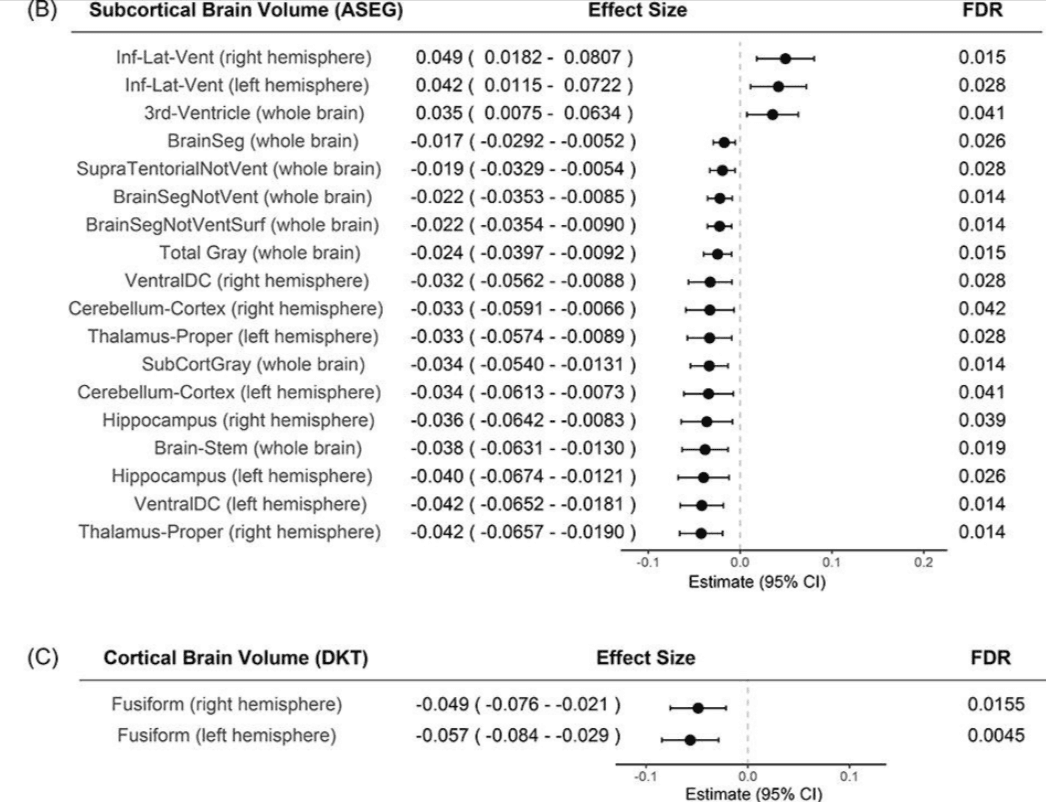
2B. IL-6 and Inflammation: Neurodegenerative Diseases
IL-6 is not just a marker in brain inflammation—it plays an active role in the development and progression of neurodegenerative diseases. Research now points to IL-6 as a driver of the inflammatory processes that underlie conditions like Alzheimer’s, Parkinson’s, and multiple sclerosis.
Take Alzheimer’s disease, for example.
In recent animal studies, researchers found that neutralizing IL-6 signaling significantly improved memory function in mice with AD-like pathology. It didn’t just improve behavior—it reversed underlying metabolic disturbances in the brain. The study highlighted that targeting IL-6 could reduce neuroinflammation, restore synaptic function, and mitigate neuronal damage, offering a new direction for Alzheimer’s interventions beyond amyloid and tau (Nature Molecular Psychiatry, 2021).
These effects are tied to IL-6’s influence on neuroinflammatory signaling cascades—particularly via its trans-signaling pathway, which fuels chronic, low-grade inflammation in neural tissue. This is distinct from IL-6’s classical signaling, which can be protective. The balance between these two modes matters deeply in the context of aging brains.
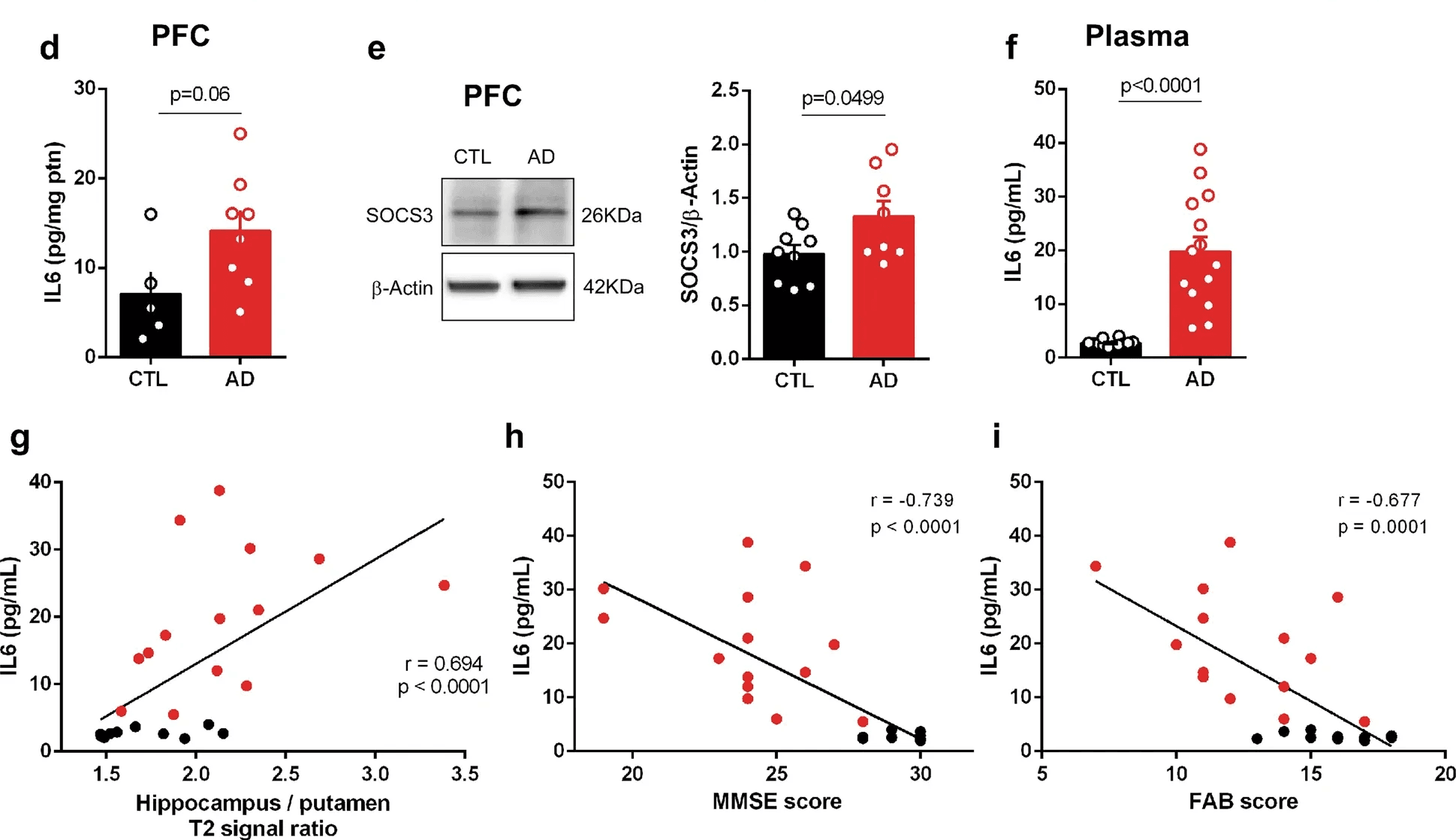
More broadly, chronically elevated IL-6 has been shown to increase blood-brain barrier permeability, allowing peripheral inflammatory molecules to enter the central nervous system. This leakage alone is enough to accelerate neurodegenerative processes. But IL-6 doesn’t stop there—it also promotes glial activation, excitotoxicity (via overactivation of NMDA receptors), and apoptosis of vulnerable neurons—all core features of diseases like Alzheimer’s, Parkinson’s, and multiple sclerosis (Frontiers in Neurology, 2020).
This is where CRP, even in its high-sensitivity form, falls short. It may reflect systemic inflammation, but it doesn’t operate within the brain. It doesn’t cross the blood-brain barrier. And it doesn’t influence neural signaling directly.
IL-6, on the other hand, operates both peripherally and centrally—and its presence in the blood may be a proxy for what’s happening in the brain before structural damage is visible on imaging.
For anyone concerned about long-term cognitive health or tracking risk in conditions like Alzheimer’s, Parkinson’s, or even age-related mild cognitive impairment, hs-IL-6 offers a more dynamic, mechanistic, and early-stage signal than CRP alone can provide.
Want to Get Ahead of Brain Inflammation?
High-sensitivity IL-6 (hs-IL-6) testing can reveal inflammation years before symptoms appear.
Most standard labs don’t offer it. Most doctors don’t test for it.
Choose between standard IL-6 and high-sensitivity IL-6 (hs-IL-6) testing—and get the full picture early.
2C. Interleukin-6 and Longevity: How Early Inflammation Predicts Aging
IL-6 isn’t just a disease marker—it’s a central signal connecting inflammation and aging—and a strong predictor of how we age. Elevated IL-6 levels have been consistently associated with earlier onset of age-related disease, loss of function, and mortality.
Large-scale research on centenarians and long-lived populations found a clear inverse relationship between plasma IL-6 levels, inflammation burden, and lifespan. Individuals with the lowest IL-6 blood test levels had the highest chance of exceptional longevity. Those with higher levels were more likely to develop cardiovascular disease, metabolic dysfunction, and cognitive decline (PLOS ONE).
Importantly, this association held even after adjusting for comorbidities and physical disability. IL-6 wasn’t just tagging along with poor health—it was acting as an upstream signal.
In a separate cohort, researchers tracked physical and cognitive performance in older adults and found that lower IL-6 and CRP levels correlated with significantly better outcomes. Participants with lower inflammation markers had stronger grip strength, better gait speed, higher cognitive test scores, and lower risk of institutionalization (Immunity & Ageing).
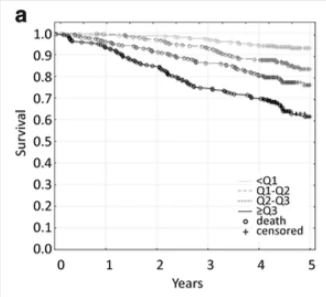
This makes IL-6 one of the few markers that links directly to both lifespan and healthspan. It reflects chronic low-grade inflammation—commonly called “inflammaging”—which drives cellular senescence, immune dysfunction, and systemic decline.
Unlike CRP, which is a downstream product of inflammatory signaling, IL-6 is a mediator. It initiates processes that disrupt metabolism, impair mitochondrial function, and accelerate neurodegeneration. That upstream role makes it more actionable: interventions that reduce IL-6 (such as anti-inflammatory diets, specific nutraceuticals, or exercise strategies that avoid overtraining) may impact disease risk more directly than targeting CRP.

In a longevity context, IL-6 is not just useful—it’s foundational. Regular testing can help catch early dysfunction, personalize interventions, and track whether anti-inflammatory strategies are working long before disease appears.
Want to see how IL-6 testing actually works—and how to interpret your results? We’ve broken it down step-by-step in our practical IL-6 test guide. It’s a great next read if you’re looking to act on what you’re learning here.
3. 🔬What Is Interleukin 6 (IL-6) and How It Differs from hs-CRP
3A. Basics of IL-6 and hs-CRP
When it comes to understanding inflammation and aging, it’s essential to know the difference between interleukin 6 (IL-6) and hs-CRP—two important, but very different, blood markers
- IL-6 (interleukin 6) is a cytokine—a signaling molecule that regulates immune activity across multiple tissues, including fat, brain, and muscle. It’s produced by a wide range of cells (immune cells, adipocytes, endothelial cells, and muscle tissue) in response to infection, injury, or stress. Importantly, IL-6 also directs the liver to produce CRP.
- hs-CRP (high-sensitivity C-reactive protein) is not a signaling molecule but a downstream byproduct. It reflects the liver’s response to IL-6 and other cytokines, acting as a broad marker of systemic inflammation.
While both are measurable in blood, their utility for health and longevity optimization differs:
- IL-6 offers early and pathway-specific information—it rises rapidly (within 1–2 hours), resolves quickly, and can reflect the source and nature of the inflammation.
- hs-CRP is slower to rise, with a longer half-life (~18–20 hours), making it useful for identifying chronic, low-grade inflammation over time.
Because IL-6 drives the inflammatory response and CRP simply marks it, testing both together provides a fuller picture: Measuring IL-6 levels through an IL-6 blood test helps identify cause and timing, while hs-CRP indicates overall burden and persistence.
This foundational distinction sets the stage for the comparisons that follow—where IL-6 demonstrates clearer tissue specificity, pathway targeting, and temporal resolution than hs-CRP.
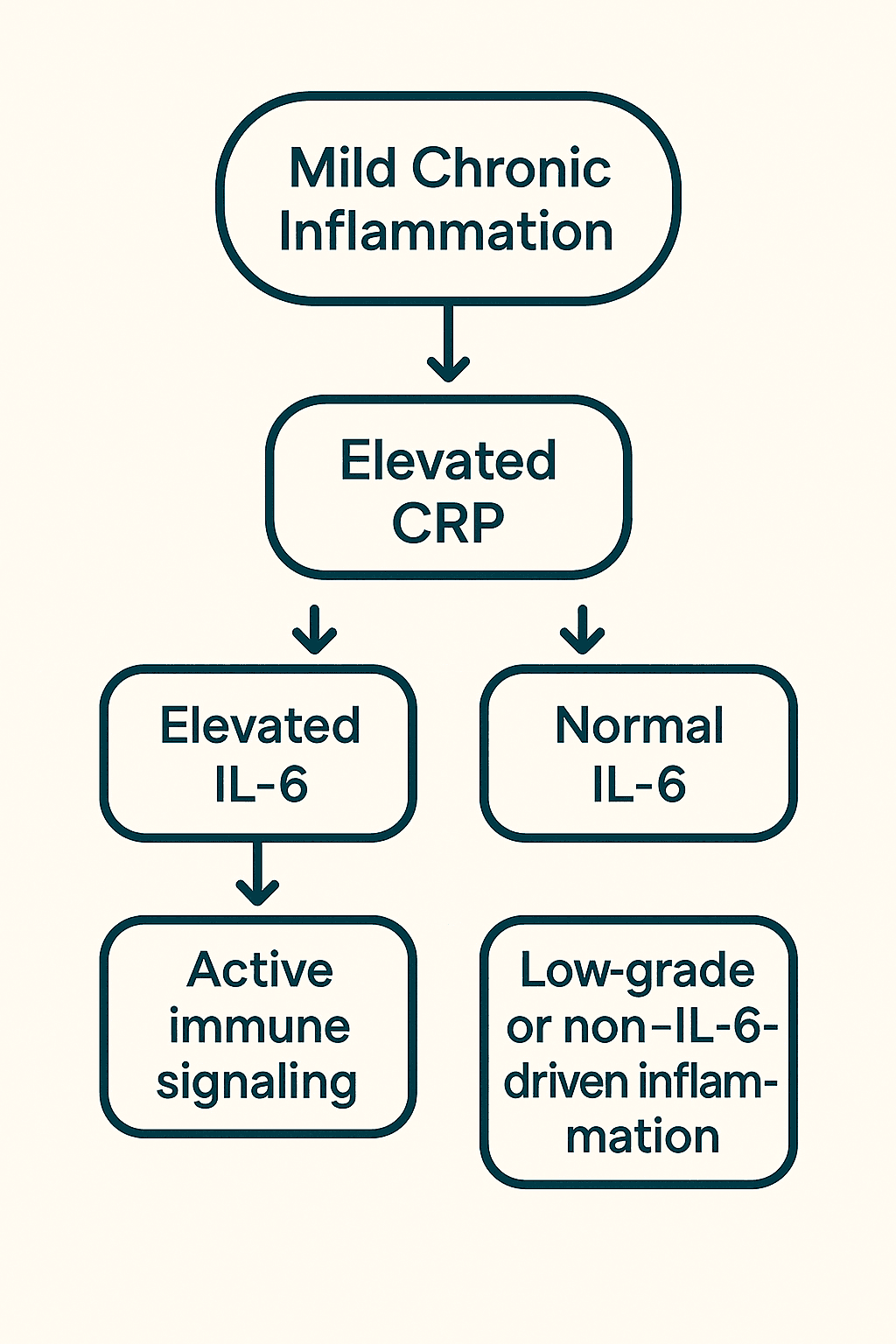
3B. IL-6, Inflammation, and Tissue-Specific Inflammation
Unlike hs-CRP, which reflects systemic inflammation without distinguishing its origin, IL-6 inflammation provides tissue-specific insight. Because IL-6 is produced locally in response to stress or damage, elevated levels can offer clues about where inflammation is occurring—if interpreted in context.
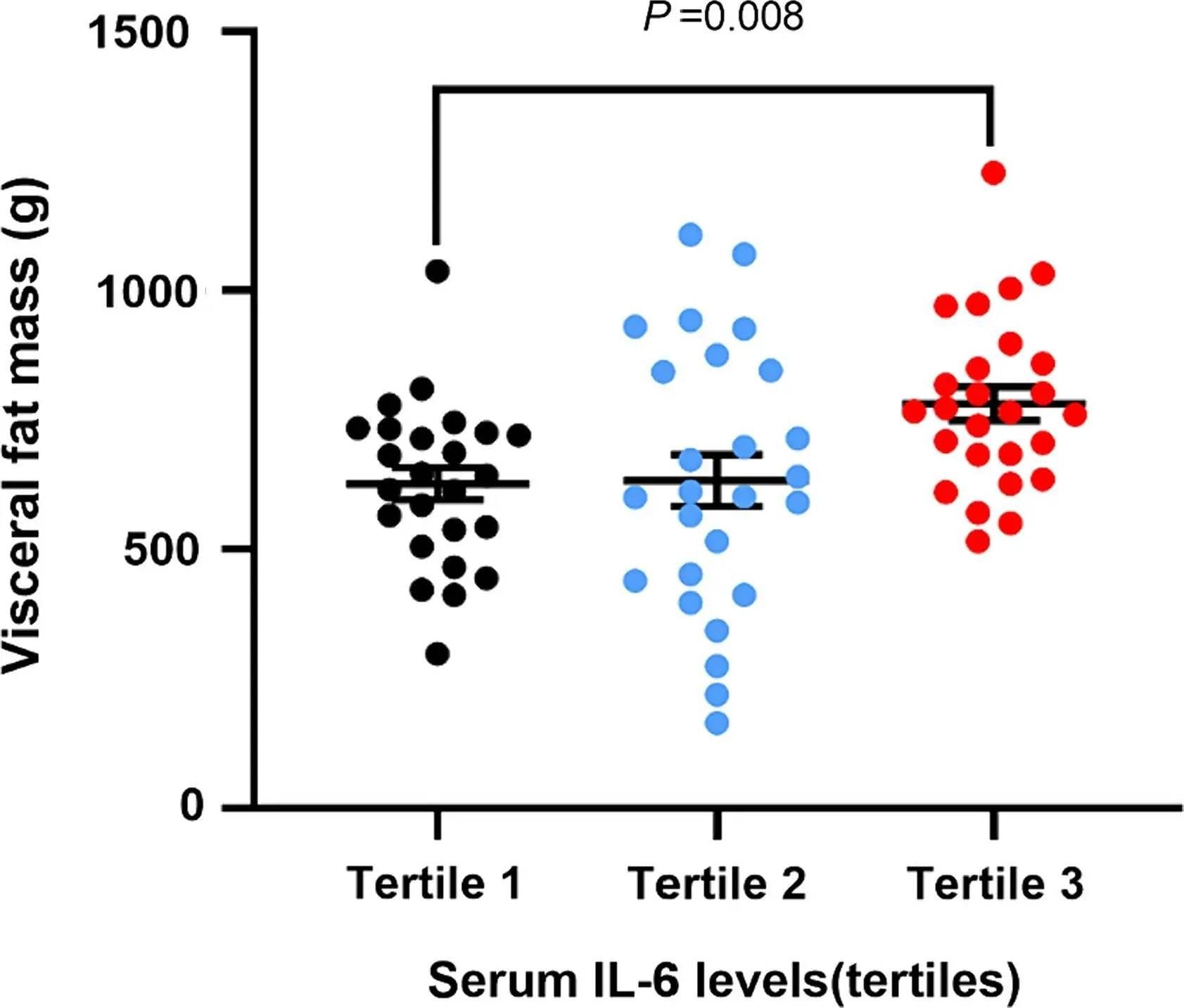
- Adipose Tissue Chronic low-grade IL-6 secretion from visceral fat is a hallmark of metabolic dysfunction and inflammation—linking IL-6 and aging through insulin resistance and systemic stress. This adipose-derived IL-6 promotes insulin resistance, impairs glucose uptake, and alters lipid metabolism—making it a key player in obesity-related diseases and type 2 diabetes. hs-CRP may also be elevated in these cases, but it doesn’t differentiate whether inflammation is coming from fat, infection, or another organ system.
- Muscle Tissue During exercise, working muscle fibers release IL-6 in large amounts. This myokine IL-6 acts in an anti-inflammatory and metabolic regulatory role—stimulating glucose uptake, fat oxidation, and downstream production of anti-inflammatory cytokines like IL-10. This physiological IL-6 spike is transient and not pathological. In contrast, CRP typically does not respond to short bouts of exercise, making it less affected by transient muscle-related changes.
- Neural Tissue Neuroinflammation is more difficult to detect in blood, but IL-6 offers a potential signal. When IL-6 is elevated alongside other neurological markers, it may point toward central nervous system inflammation, particularly in neurodegenerative contexts like Alzheimer’s. hs-CRP does not cross the blood-brain barrier and has no role in neuroimmune signaling, making it poorly suited to track brain-specific inflammation (Nature Molecular Psychiatry).
Get Weekly Insights to Personalize Your Own Longevity Roadmap
In short, IL-6 levels—when interpreted alongside timing, symptoms, and co-markers—can offer directional insight into which tissue system is inflamed. hs-CRP, though useful for monitoring general burden, lacks this level of resolution.
BTW—if you’ve ever trained hard, eaten clean, and still carried stubborn belly fat, IL-6 might be the missing piece. Here’s how it rewires fat storage and fat burning → Visceral Fat and IL-6: The Longevity Risk Most Men Miss
Examples of IL-6–Associated Tissue Inflammation and Co-Markers
| Possible Source | Supporting Co-Markers | What It Suggests |
|---|---|---|
| Adipose Tissue (Fat) | Leptin, Adiponectin (↓), Resistin | Visceral fat-driven inflammation, insulin resistance |
| Skeletal Muscle | Creatine kinase (CK), Myoglobin | Muscle stress or damage (exercise, injury) |
| Brain / Neural Tissue | Neurofilament light chain (NfL), GFAP | Neuroinflammation or early neurodegeneration |
| Chronic Infection | Elevated WBCs, hs-CRP + IL-6, Pathogen-specific IgGs | Low-grade or persistent immune activation |
| Autoimmune Activation | IL-6 + ANA, RF, or anti-dsDNA | Potential early-stage autoimmune signaling |
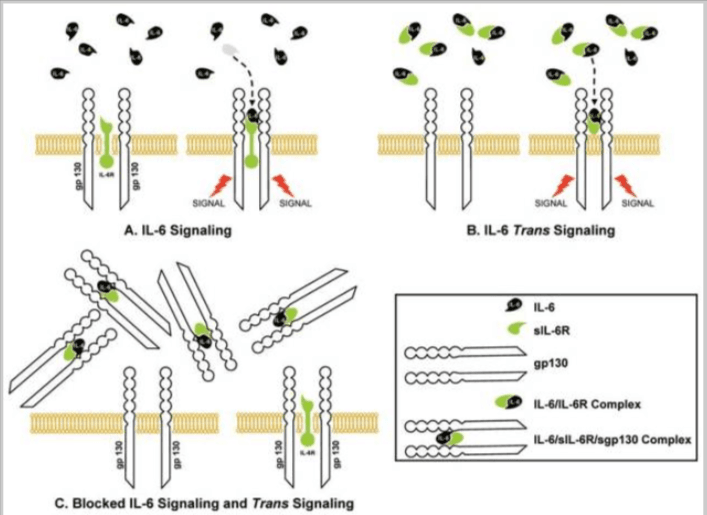
3C. IL-6 Signaling Pathways: Classical vs. Trans-Inflammation
Understanding how IL-6 drives inflammation is critical. IL-6 operates through two distinct signaling pathways—classical and trans-signaling—and this distinction shapes whether its effects are regenerative or pro-inflammatory.
- Classical Signaling This pathway involves IL-6 binding to membrane-bound IL-6 receptors found on limited cell types, including hepatocytes and some leukocytes. Classical signaling tends to support homeostatic and regenerative processes, such as tissue repair and anti-inflammatory feedback. In short, not all IL-6 activity is harmful.
- Trans-Signaling This mode of action occurs when IL-6 binds to a soluble IL-6 receptor (sIL-6R), which then interacts with gp130 receptors present on most cell types. This broadens IL-6’s reach and shifts it toward pro-inflammatory activity—driving chronic inflammation, endothelial dysfunction, and immune dysregulation.
This complexity is one reason why measuring IL-6 inflammation provides more pathway-specific insight than relying solely on systemic markers like hs-CRP.
CRP, has no pathway specificity. It’s a passive downstream marker and does not distinguish whether inflammation is regenerative or destructive. IL-6, when interpreted with context or co-markers, offers this added resolution.
3D. How IL-6 (interleukin 6) and CRP React Over Time: Fast vs. Slow Inflammatory Markers
One of the most important differences between IL-6 and CRP is how quickly they respond to—and resolve after—inflammatory events. Understanding these timelines is key when using an IL-6 blood test for early detection of inflammation and aging risk
- IL-6 inflammation rises within 1–2 hours of an immune trigger and can peak within 4–6 hours. It resolves rapidly, making it useful for identifying acute or transient inflammatory episodes, especially those that CRP may entirely miss. This includes inflammatory responses to training, early-stage infections, or meal-induced immune responses.
- hs-CRP, with a half-life of 18–20 hours, responds more slowly (often peaking 12–24 hours after the inflammatory signal) and remains elevated longer—even after the triggering event has subsided. This makes it a more stable marker of chronic inflammation, but one that lacks fine-grained temporal resolution.
This difference means IL-6 can capture fleeting physiological changes (which might be either beneficial or harmful), while hs-CRP is better suited for tracking persistent inflammatory burden. Using them together can help differentiate between transient immune activation and sustained inflammatory stress.
Because IL-6 moves faster, it offers a chance to detect subtle inflammatory changes earlier—an important advantage for anyone focused on proactive longevity strategies.
Key Differences Between IL-6 and CRP as Inflammatory Markers:
| Characteristic | IL-6 | CRP |
|---|---|---|
| Type | Upstream signaling cytokine | Downstream acute-phase protein |
| Response Timing | Early marker (rises within 1–2 hours after trigger) | Later marker (peaks 12–24 hours after trigger) |
| Production Site | Produced by multiple cell types (immune cells, adipocytes, endothelial cells) | Produced by the liver in response to IL-6 signaling |
| Half-Life | Shorter (2–4 hours) | Longer (18–20 hours) |
| Specificity | More specific to active inflammatory pathways (can reflect tissue-specific activity) | General marker of systemic inflammation |
Now that you understand how IL-6 and CRP behave differently, the next step is knowing how to use IL-6 testing strategically—whether your CRP is normal, elevated, or somewhere in between.
FAQ
What does IL-6 do to the brain?
IL-6 triggers inflammation in the brain, disrupting the blood-brain barrier and promoting neuronal damage.
Chronically elevated IL-6 is associated with cognitive decline, memory loss, and higher risk for neurodegenerative diseases like Alzheimer’s.
Because IL-6 rises before structural brain changes occur, testing IL-6 offers one of the earliest ways to catch brain inflammation while it’s still reversible.
Does inflammation increase aging?
Yes, chronic inflammation accelerates biological aging by damaging cells, impairing metabolism, and disrupting brain function.
Elevated IL-6 is a key signal of this “inflammaging” process, linking directly to earlier onset of cardiovascular disease, diabetes, dementia, and frailty.
Keeping IL-6 low is one of the most actionable strategies for extending both lifespan and healthspan.
What are the diseases associated with IL-6?
Persistently high IL-6 is linked to cardiovascular disease, Alzheimer’s disease, Parkinson’s disease, type 2 diabetes, rheumatoid arthritis, and certain cancers.
Even before these diseases develop, elevated IL-6 indicates inflammatory stress that can erode health over years.
Testing IL-6 can reveal hidden risks early enough to act on them.
What happens if interleukin-6 is high?
High interleukin-6 means active inflammation is underway, even if no symptoms are visible yet.
It can signal early metabolic dysfunction, brain inflammation, vascular damage, or immune system imbalance—often years before clinical disease appears. Getting an IL-6 blood test provides a critical window to intervene early.
What triggers IL-6 production?
IL-6 is triggered by infection, injury, chronic visceral fat, blood sugar dysregulation, stress, and neuroinflammation.
It also rises temporarily after intense exercise, but normally returns to baseline within 24 hours.
Persistent elevation outside of acute stress signals deeper inflammatory problems—and warrants investigation.
BTW—if you want to see how IL-6 links to metabolic issues like visceral fat, even in lean people, this guide breaks it down → Visceral Fat and IL-6 Explained
Is IL-6 an inflammatory marker?
Yes, IL-6 is a core inflammatory marker that rises early in the immune response.
Unlike downstream markers like CRP, IL-6 reflects active tissue inflammation and helps drive the inflammatory cascade.
Because it rises before damage becomes obvious, IL-6 is an essential target for longevity-focused health optimization.
What are the risks of IL-6?
High IL-6 increases risk for heart attacks, strokes, dementia, type 2 diabetes, and autoimmune disease.
Even moderate elevations contribute to accelerated aging and cognitive decline.
Identifying and addressing high IL-6 blood test result early can shift long-term health outcomes.
Is IL-6 inflammatory or anti-inflammatory?
IL-6 can act as both, depending on how it signals.
Through classical pathways, IL-6 supports healing and immune regulation.
Through trans-signaling, it drives chronic inflammation, tissue damage, and disease.
When IL-6 remains elevated over time, its inflammatory effects dominate—and contribute directly to accelerated aging and disease risk.
📈 IL-6, Inflammation, and Longevity: Why It Belongs on Your Radar
When it comes to optimizing for longevity, inflammation control, and brain health, IL-6 isn’t just another lab value — it’s an early signal that can change your aging trajectory long before symptoms appear.
Unlike CRP, which tells you that inflammation has already happened, IL-6 gives you a glimpse into what’s happening now — at the level of tissue-specific immune signaling, neural health, metabolic stress, and vascular risk. If you’re serious about staying ahead of aging, reducing inflammation and aging risk, and tailoring your interventions more precisely, IL-6—and the use of a strategic IL-6 blood test—should be on your shortlist of essential biomarkers.
In the next article, we’ll break down exactly how to use IL-6 testing in practice:
Who should measure it, how to think about your results, and how it can help you build a smarter and more personalized longevity strategy.
Learn how to use IL-6 testing strategically in Part 2: Early Detection, Better Decisions.
References
- Bradburn S, Sarginson J, Murgatroyd CA. Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Front Aging Neurosci. 2018 Jan 8;9:438. doi: 10.3389/fnagi.2017.00438. PMID: 29358917; PMCID: PMC5766662.
- Ferreira JP, Vasques-Nóvoa F, Neves JS, Zannad F, Leite-Moreira A. Comparison of interleukin-6 and high-sensitivity C-reactive protein for cardiovascular risk assessment: Findings from the MESA study. Atherosclerosis. 2024 Mar;390:117461. doi: 10.1016/j.atherosclerosis.2024.117461. Epub 2024 Jan 24. PMID: 38306764.
- Mehta NN, deGoma E, Shapiro MD. IL-6 and Cardiovascular Risk: A Narrative Review. Curr Atheroscler Rep. 2024 Nov 26;27(1):12. doi: 10.1007/s11883-024-01259-7. PMID: 39589436; PMCID: PMC11599326.
- Zhao Z, Zhang J, Wu Y, Xie M, Tao S, Lv Q, Wang Q. Plasma IL-6 levels and their association with brain health and dementia risk: A population-based cohort study. Brain Behav Immun. 2024 Aug;120:430-438. doi: 10.1016/j.bbi.2024.06.014. Epub 2024 Jun 17. PMID: 38897328.
- Lyra e Silva, N.M., Gonçalves, R.A., Pascoal, T.A. et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl Psychiatry 11, 251 (2021). https://doi.org/10.1038/s41398-021-01349-z
- Parks EE, Logan S, Yeganeh A, Farley JA, Owen DB, Sonntag WE. Interleukin 6 reduces allopregnanolone synthesis in the brain and contributes to age-related cognitive decline in mice. J Lipid Res. 2020 Oct;61(10):1308-1319. doi: 10.1194/jlr.RA119000479. Epub 2020 Jul 15. PMID: 32669383; PMCID: PMC7529050.
- Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, et al. (2009) IL-6 Mediated Degeneration of Forebrain GABAergic Interneurons and Cognitive Impairment in Aged Mice through Activation of Neuronal NADPH Oxidase. PLoS ONE 4(5): e5518. https://doi.org/10.1371/journal.pone.0005518
- Puzianowska-Kuźnicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, Slusarczyk P, Skalska A, Jonas M, Franek E, Mossakowska M. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing. 2016 Jun 3;13:21. doi: 10.1186/s12979-016-0076-x. PMID: 27274758; PMCID: PMC4891873.
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006 Jun;61(6):575-84. doi: 10.1093/gerona/61.6.575. PMID: 16799139; PMCID: PMC2645627.