You’ve probably heard of memory loss or brain fog.
But what about brain shrinkage?
MRI studies show that even in healthy, high-performing adults, the brain starts to lose volume—especially in regions tied to focus, planning, and emotional regulation—as early as your 30s and 40s.
Get Weekly Sleep Insights In Your Inbox. Beyond Melatonin, Blue Light, and Caffeine.
The cortex starts to thin.
Frontal regions lose volume.
And while aging plays a role, there’s another, less obvious driver behind these early changes: sleep quality.
Poor sleep was associated with advanced brain age in midlife.
— Dr. Clémence Cavaillès, UCSF, 2024
New research shows that poor sleep fragmentation, poor REM sleep, or misaligned sleep may be one of the most underrecognized causes of brain shrinkage in midlife.
And it doesn’t take extreme deprivation to matter.
Even subtle disruptions can lead to measurable cortical atrophy over time—undermining both cognitive longevity (your capacity to think, focus, and sustain brain health over decades) and performance longevity (your ability to maintain physical, mental, and emotional output as you age).
This article examines how poor sleep contributes to measurable brain shrinkage—with MRI evidence from clinical and population studies—and defines what truly restorative sleep looks like, according to sleep medicine standards.
It also shows why most consumer sleep trackers miss the biological signals that matter most for long-term brain health.
➤ How fragmented or REM-poor sleep accelerates structural brain decline
➤ Which regions are affected—and how early the changes begin
➤ What defines restorative sleep (hint: it’s not your ring score)
➤ The overlooked sleep disruptor most people never check for
Table of Contents
Section 1: Poor Sleep Quality Triggers Cortical Atrophy, Brain Shrinkage and Brain Aging
Sleep isn’t just recovery—it’s structural maintenance & repair.
When sleep is fragmented or misaligned, the brain’s nightly repair cycles falter. Over time, that disruption becomes one of the earliest brain shrinkage causes—contributing to cortical atrophy, or thinning of the outer brain layers that govern executive function, emotional regulation, and attention.
MRI data now show these changes can emerge in midlife, even in cognitively normal adults.
And the common thread across studies isn’t total sleep time—it’s sleep quality: how continuous, coordinated, and neurophysiologically complete the sleep process is.
➤ The UCSF/CARDIA Study – Poor Midlife Sleep and Brain Shrinkage on MRI
In a 2024 longitudinal study from UCSF, researchers followed nearly 600 adults (mean age 40.4) over a 15-year span, investigating how common midlife sleep disturbances—influence brain aging. The sleep disturbances they examined include:
- ▪️ Bad sleep quality (”SQ”)
- ▪️ Difficulty initiating and maintaining sleep (”DIS” and “DMS”)
- ▪️ Early morning awakening (”EMA”)
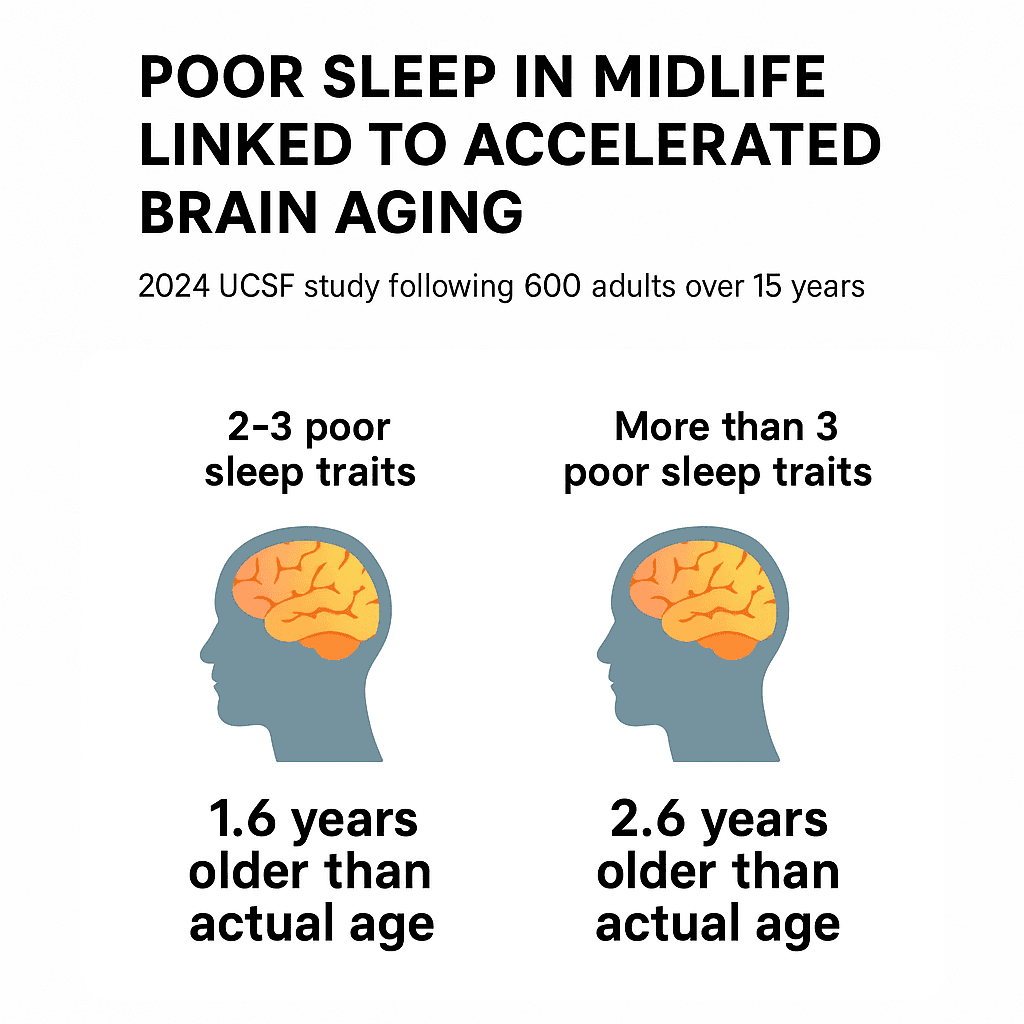
More poor sleep traits = older brain age, even in healthy midlife adults.
These weren’t cases of severe sleep loss or diagnosed disorders—but rather the kind of persistent midlife sleep difficulties that often go unaddressed.
When researchers compared sleep patterns with follow-up brain MRIs, the findings revealed a clear, dose-dependent relationship between poor sleep and brain aging:
- Participants with 2–3 poor sleep traits—such as difficulty falling asleep, waking frequently, or rising too early—had brains that appeared 1.6 years older than their actual age.
- Those with more than 3 poor sleep traits showed even more pronounced aging, with brains that appeared 2.6 years older.
So how did they determine the brain age of the study participants?
They estimated “brain age” by comparing each participant’s MRI data to patterns seen in a normative dataset of healthy individuals. The machine learning models had been trained to recognize how brain structure typically changes with age—such as brain shrinkage from thinning of the cortex or loss of volume in specific regions like the prefrontal cortex and hippocampus.
By measuring how closely each participant’s brain matched these age-related patterns, the model generated an estimated “brain age.” The difference between this and their actual age—called the brain age gap—served as a marker of accelerated or preserved brain aging.
Participants with poor midlife sleep had higher brain age gaps, reflecting more advanced structural atrophy than expected for their chronological age.
Importantly, these findings held even after controlling for a wide range of potential confounding variables:
- ▪️ Sociodemographic factors: age, sex, and years of education
- ▪️ Lifestyle and health behaviors: physical activity, smoking status, alcohol use, and BMI
- ▪️ Cardiometabolic risk markers: hypertension, diabetes, and cholesterol levels
- ▪️ Mental health indicators: including depressive symptoms
- ▪️ Sleep quantity: total sleep duration and time in bed
By adjusting for these variables, the researchers were able to isolate sleep quality itself as a likely driver of accelerated brain aging.
“Even if the cause of dementia is unrelated to sleep, poor sleep may advance or exacerbate cognitive symptoms.”
— Dr. Clémence Cavaillès, UCSF 2024
Taken together, the results suggest that persistent midlife sleep disturbances may reflect more than lifestyle—they may signal early physiological breakdowns. Dysregulation in circadian timing, HPA-axis activity, or synaptic recovery may contribute directly to structural brain changes that precede clinical cognitive impairment.
By the way, If you’ve been following my work on hormones and sleep, you’ll know how much depth there is beneath the surface.
If you’re ready to go deeper and take a systems-based approach to improving your sleep, Sleep OS Hormones is now available as a 60-day self-guided program with dedicated systems for estrogen, progesterone, and testosterone, or bundled together for a more complete approach.
or
➤ Neurology 2014 – Cortical Atrophy Tracks with Poor Sleep Efficiency
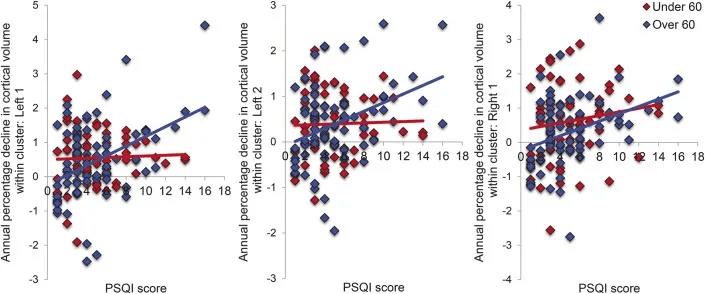
A longitudinal study published in Neurology reinforces the biological impact of poor sleep. Researchers followed 147 community-dwelling adults (age 53.9 ± 15.5 years) for 3.5 years, measuring sleep quality with the Pittsburgh Sleep Quality Index (PSQI) and tracking changes in brain structure and brain shrinkage on MRI.
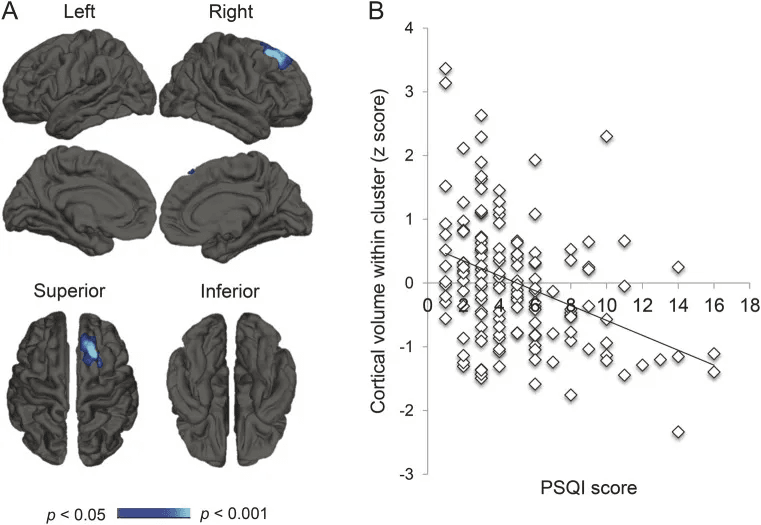
Their findings:
- Lower sleep efficiency was associated with reduced volume in the right superior frontal cortex.
- Over time, poor sleep quality predicted progressive cortical atrophy across frontal, temporal, parietal, and cingulate regions.
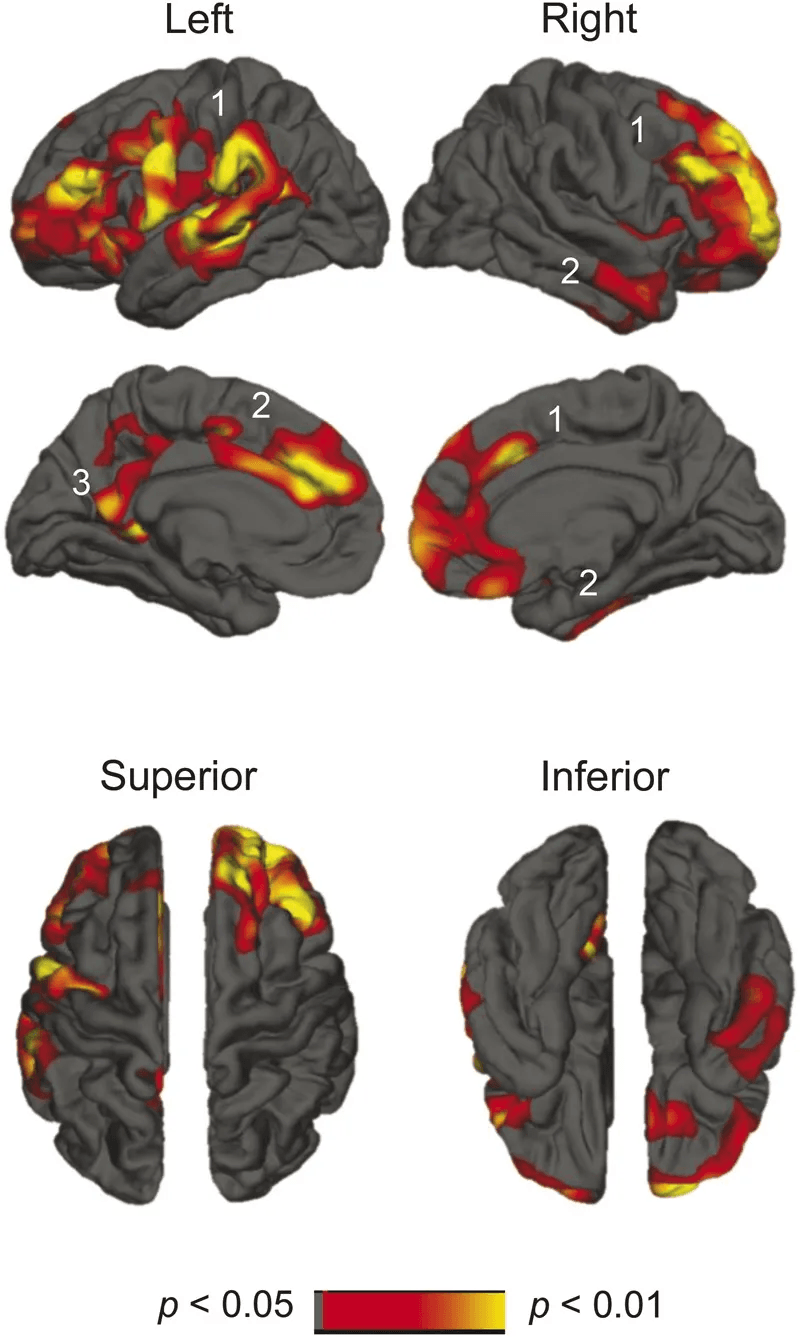
These areas are not memory centers.
They govern executive function, mood regulation, behavioral control, and social cognition. The observed atrophy follows a frontal lobe-predominant pattern—similar to what’s seen in the early stages of neurodegenerative conditions. In many cases, these structural changes appear before hippocampal shrinkage or measurable memory decline.
Longitudinal measures of cortical atrophy were widely correlated with sleep quality. Poor sleep may be a cause or a consequence of brain atrophy.
— Claire Sexton, 2014
Importantly, these effects remained after adjusting for physical activity, cardiovascular health, and BMI—reinforcing sleep quality as an independent factor.
Mechanistically, poor sleep disrupts the brain’s regenerative capacity. Impaired slow-wave signaling weakens glial-driven repair, compresses synaptic recovery windows, and reduces interregional synchrony. This cumulative dysfunction is now considered a likely contributor to early-stage brain shrinkage causes—not just a symptom, but a driver of long-term structural decline.
This is why sustained, unfragmented sleep isn’t just a wellness target—it’s a foundational requirement for long-term neurological integrity, cognitive longevity (your brain’s structural and functional resilience), and performance longevity (your ability to sustain focus, emotional control, and decision-making under real-world demands).
Section 2: REM Sleep Loss and Brain Shrinkage in Alzheimer’s-Prone Regions
REM sleep—the phase marked by rapid eye movement, vivid dreaming, and heightened brain activity—is often underestimated in discussions of cognitive longevity. Yet it plays a non-redundant, biologically distinct role in maintaining both the structure and function of the brain.
Unlike slow-wave sleep, REM facilitates emotional memory integration, synaptic pruning, and network recalibration. And critically, it helps stabilize regions most vulnerable to Alzheimer’s disease.
Loss of REM sleep doesn’t just affect how we feel the next day—it can initiate structural decline in default mode network hubs, accelerating brain shrinkage in ways that standard sleep metrics don’t always account for.
Sleep deficiency is associated with the atrophy of the inferior parietal region, which is observed in early AD. Sleep architecture may be a modifiable risk factor for AD.
– Gawson Cho, Yale School of Medicine
➤ Brain Shrinkage Causes in Alzheimer’s-Vulnerable Regions: REM Sleep
A 2025 study from Yale School of Medicine followed 271 older adults over 13–17 years, combining polysomnography (in-lab sleep architecture testing) with longitudinal MRI scans to investigate sleep’s role in structural brain aging.
Their findings pointed to a key mechanism of interest: reduced REM sleep as a potential contributor to brain shrinkage causes in Alzheimer’s-vulnerable regions.
- ▪️ Lower REM sleep was significantly associated with smaller volumes in the inferior parietal lobule (β = –75.52 mm³) and precuneus (β = –31.93 mm³)—two high-priority regions in early Alzheimer’s progression.
- ▪️ Slow-wave sleep (SWS) was also evaluated, with reductions showing smaller—but still meaningful—associations: notably in the entorhinal cortex and medial prefrontal areas, regions involved in memory encoding and metabolic clearance.
These associations remained statistically significant even after adjusting for a wide spectrum of confounders:
- Demographics: Age, sex, years of education
- Genetics: APOE4 genotype (a known Alzheimer’s risk marker)
- Brain structure controls: Intracranial volume
- Sleep metrics: Total sleep time (TST), apnea-hypopnea index (AHI)
- Cognition: Baseline global cognitive function
- Vascular/lifestyle: Alcohol use, smoking (pack-years), hypertension, coronary heart disease, diabetes, and obesity
What’s interesting is not just the volume loss—but where it occurs.
The precuneus and inferior parietal lobule are central hubs in the default mode network (DMN)—a system implicated in self-referential thought, memory retrieval, and attentional control. These regions are among the first to show shrinkage in Alzheimer’s, often before any hippocampal changes or detectable memory symptoms.
While both REM and slow-wave sleep (SWS) were examined, REM sleep showed a more robust and region-specific relationship with cortical atrophy—suggesting its unique role in preserving the structural integrity of Alzheimer’s-vulnerable brain networks.
This points to REM-associated atrophy as a potential early biomarker of disrupted network integrity—not just a byproduct of aging, but a modifiable driver of long-term brain vulnerability.
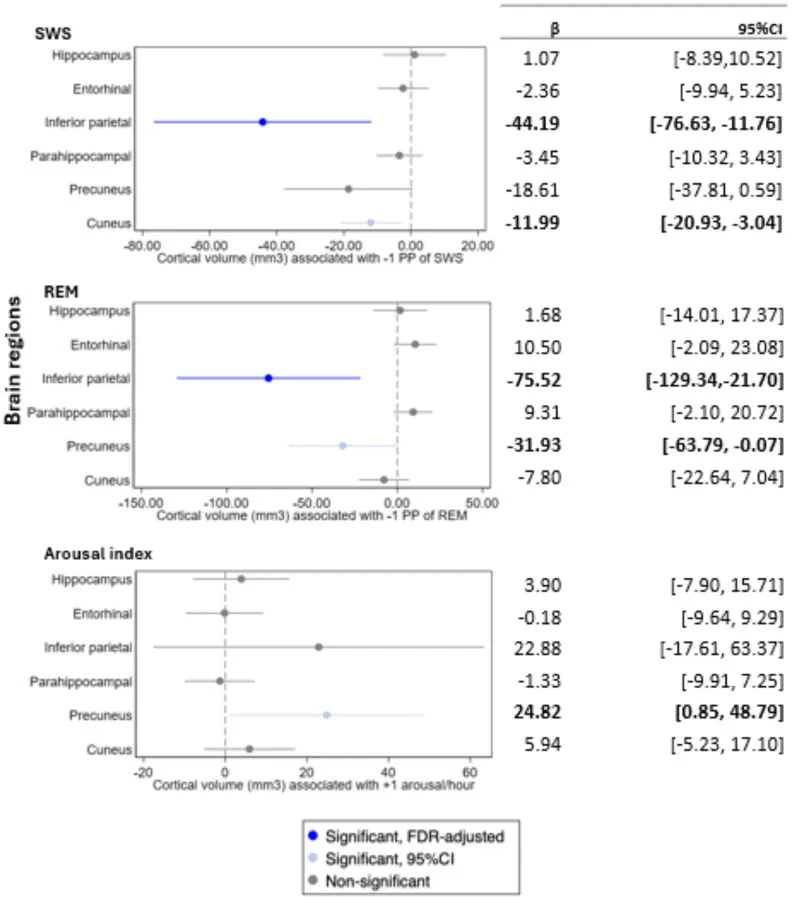
Bold face indicates statistical significance based on 95% confidence intervals.
➤ Circadian Misalignment of REM Sleep and Microstructural Brain Shrinkage
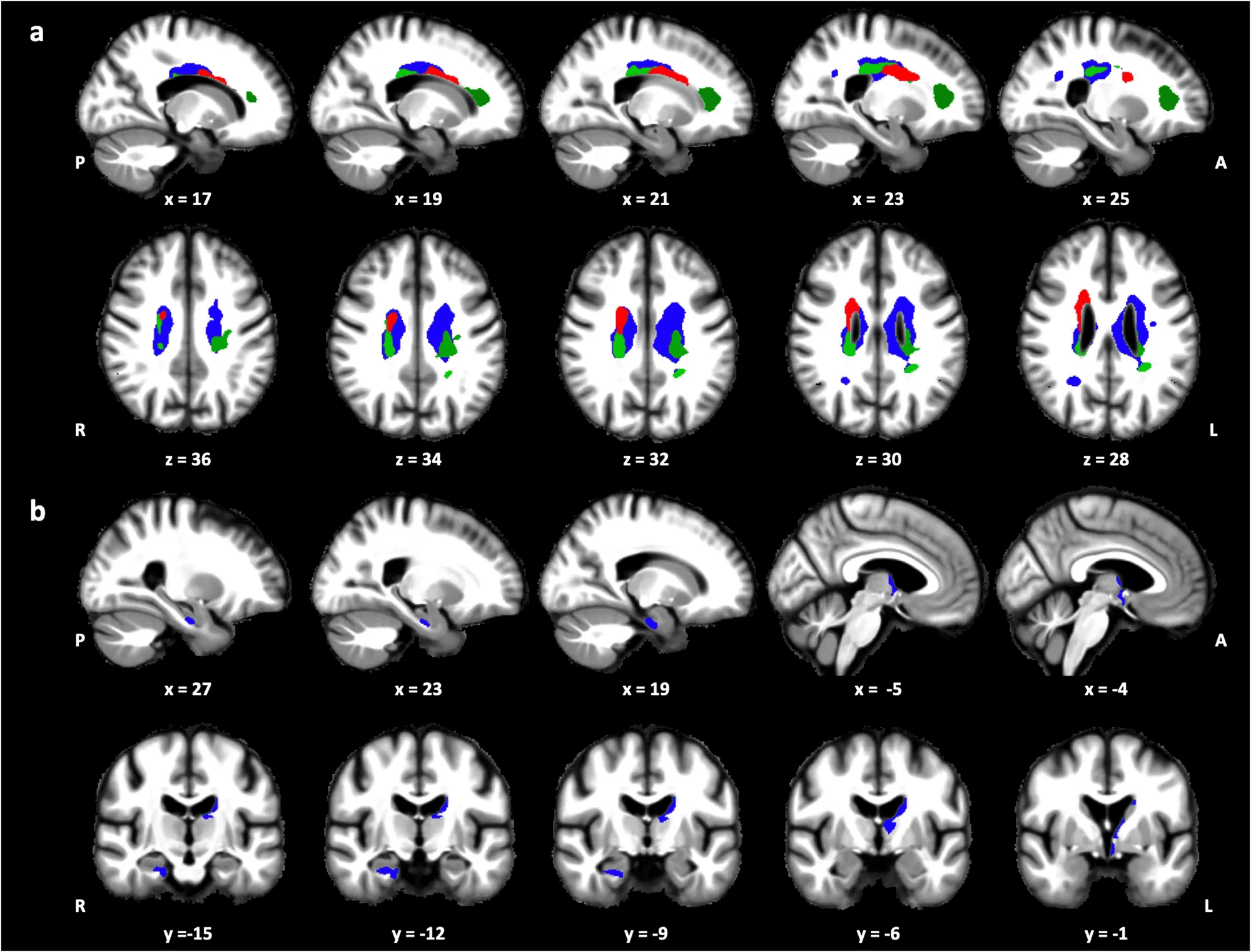
A 2024 study by Deantoni et al. (Communications Biology) introduced a novel lens on sleep-brain dynamics: circadian amplitude of REM sleep—how well REM expression aligns with circadian timing, not just how much REM sleep occurs.
Using a 40-hour multiple-nap protocol in tightly controlled conditions, researchers tracked how REM varied across internal biological time. They then correlated this with MRI-based microstructural markers, including magnetization transfer saturation (MTsat) and R1 values—which reflect myelin density, neurite integrity, and cellular microstructure.
Their findings were striking:
- Lower circadian REM amplitude predicted lower MTsat and R1 in major white matter tracts, indicating myelin loss and axonal disruption.
- In gray matter, reduced R1 values appeared in the hippocampus, thalamus, hypothalamus, and parahippocampus—regions central to memory, stress regulation, and sleep-wake control.
- Most of the affected white matter clustered in periventricular zones—already known for vulnerability to vascular aging, glial reactivity, and inflammatory burden.
Importantly, these structural signatures are sub-visible on standard MRI. MTsat and R1 detect subtle biological decline before volumetric shrinkage emerges, offering early insight into brain resilience loss.
Our findings highlight the circadian regulation of REMS as a neurophysiological correlate of subtle brain microstructural changes, including demyelination and decreased non-neuronal cell and/or neurite density…
Why Circadian Misalignment of REM Sleep Matters for Cognitive Longevity & Performance Longevity:
- REM-rich cycles concentrate in the final third of the night, when modern sleep is most likely to be truncated by alarms, late light, alcohol, or erratic schedules.
- Circadian misalignment here doesn’t just shorten REM—it shifts when it occurs, disrupting limbic pruning, synaptic recalibration, parasympathetic rebalancing, and glymphatic clearance.
- Over time, this timing mismatch may impact the brain’s recovery capacity—even in the absence of overt sleep deprivation—adding to the list of brain shrinkage causes that compromisie cognitive longevity by degrading the networks that support attention, memory integration, and emotional regulation.
- ▪️The impact also extends to performance longevity: when REM fails to align with circadian cues, the brain’s resilience under stress, adaptability, and executive control begin to decline, even if total sleep hours seem adequate.
This study deepens a key insight: sleep quality isn’t just about depth or duration—it’s about circadian timing. To preserve brain structure, REM must not only occur—it must occur in rhythm.
Section 3: Night-Time Awakenings and Cortical Atrophy (Cortical Thinning)
When it comes to brain aging, how continuously we sleep may matter as much as how long.
Sleep fragmentation—defined as repeated micro-awakenings that disrupt the architecture of sleep—has emerged as a risk factor for brain shrinkage causes. Unlike sleep deprivation, fragmentation doesn’t always affect how we feel the next day. But its impact is often delayed and overlooked.
Each brief awakening pulls the brain out of deeper sleep stages. That interruption resets processes critical to overnight maintenance: synaptic homeostasis, emotional regulation, and glymphatic waste clearance.m
Over time, these nightly disruptions may contribute to cortical atrophy—particularly in regions responsible for behavioral control and emotional flexibility.
➤ The 2016 Sleep Study – Sleep Fragmentation and Frontal Cortical Atrophy
Lim et al. (2016) studied 141 older adults using actigraphy—a wrist-worn motion sensor that records subtle movements during sleep—to quantify sleep fragmentation across seven consecutive nights.
Unlike sleep questionnaires, actigraphy does not rely on memory or self-report. It detects sleep fragmentation from restlessness and brief arousals that break sleep continuity, offering a more objective view of sleep architecture.
After data collection, participants underwent MRI scans to assess cortical gray matter volume across multiple brain regions.
These were their findings:
- Higher sleep fragmentation was associated with lower gray matter volume in the lateral orbitofrontal cortex and the inferior frontal gyrus pars orbitalis—bilaterally. ▪️
- These effects held after accounting for a wide range of covariates, including age, sex, education, depression symptoms, medical comorbidities, and baseline cognitive scores.
These adjustments strengthen the interpretation that sleep disruption itself—rather than pre-existing cognitive decline—was linked to cortical atrophy.
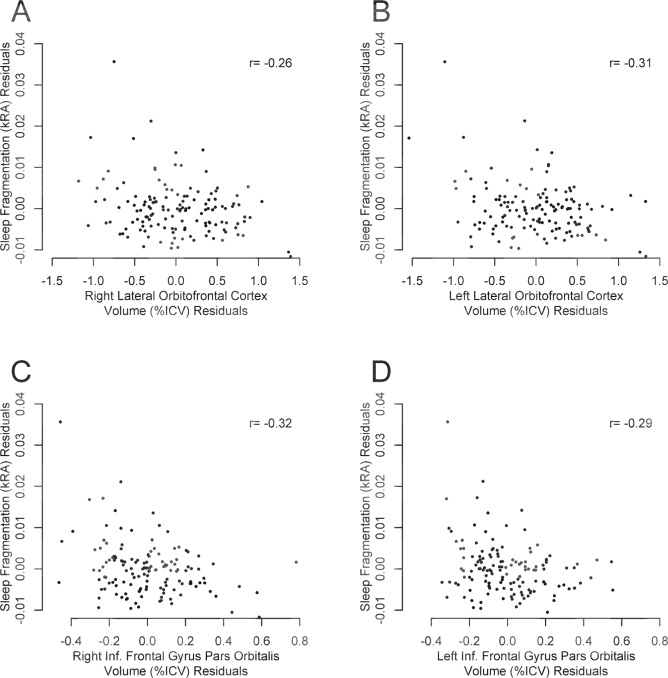
The affected regions are key to emotional self-regulation, decision-making, and inhibitory control—functions that often decline early in frontotemporal and executive-type dementias.
What’s scientifically notable is that these volume losses occurred in people without overt cognitive impairment or stroke, and not using sleep medications.
Taken together, the study supports two possibilities: that sleep fragmentation may be an early, independent contributor to cortical atrophy—or that subtle loss of frontal structure destabilizes sleep-wake regulation in aging brains.
➤ Why Sleep Fragmentation Is Easy to Miss—and Hard to Repair
You don’t need to be sleep-deprived to experience disrupted sleep.
Even with a full night in bed, brief, recurrent awakenings—often too short to register—can fragment sleep architecture and interrupt core neurophysiological processes. Sleep may appear sufficient by duration, yet remain compromised in function.
This makes fragmentation difficult to detect.
Many commercial devices prioritize total hours, not continuity.
But as the brain ages, uninterrupted sleep becomes increasingly essential. Small disturbances—often from physiological or environmental factors—can displace the brain from slow-wave sleep, where most restorative activity occurs.
These interruptions are frequently linked to:
- Nocturnal urination (nocturia)
- Obstructive or central sleep apnea
- Environmental light, noise, or temperature
Critically, these events rarely result in full wakefulness. Yet even momentary arousals—long enough to shift brain states—can suspend synaptic downscaling, emotional memory consolidation, and glymphatic waste clearance.
Because the effects manifest biologically rather than perceptually, they’re easy to misattribute. Morning fatigue, reduced clarity, or mood variability may be seen as stress or poor habits. In reality, they may reflect incomplete neural recalibration.
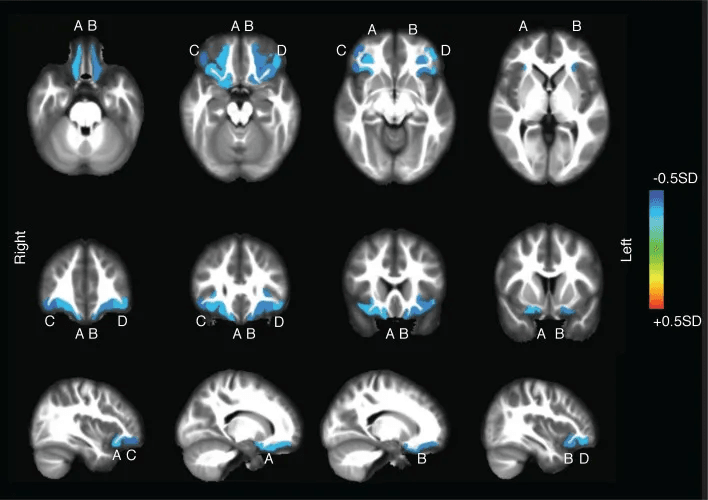
Unlike REM, which is typically delayed by sleep truncation, slow-wave sleep dominates the first half—which means even light exposure, metabolic issues, or room temperature shifts in the early night can affect its depth & duration.
Once the brain exits a slow-wave phase prematurely, the opportunity for repair may not return that night.
This fragility makes early-night stability especially important—and helps explain why some of the most meaningful interventions for cognitive aging may begin not with more sleep, but with more continuous sleep.
By the way, are you struggling with fragmented sleep—and wondering why melatonin isn’t helping? Here’s why melatonin often fails to deliver that continuity—and what to do instead.
Section 4: What Good Sleep Really Means (And How to Know If You’re Getting It)
➤ Your Sleep Ring Isn’t Telling You Everything
Most people rely on wearables to assess their sleep—but these devices overestimate sleep quality and misclassify stages with surprising regularity.
Studies now show that sleep stage classification accuracy across popular wearables ranges from just 50% to 86% compared to clinical-grade polysomnography (PSG)—the gold standard for measuring sleep architecture.
One example: the Apple Watch Series 8 was found to overestimate light sleep by an average of 45 minutes per night, while underestimating deep sleep by 43 minutes.
For sleep stage classification, sensitivity ranged from 50% to 86%.
– Robbins et al., 2024, Division of Sleep Medicine, Harvard Medical School
Oura Ring: sensitivity 76.0–79.5%, precision 77.0–79.5%
Fitbit: sensitivity 61.7–78.0%, precision 72.8–73.2%
Apple: sensitivity 50.5–86.1%, precision 72.7–87.8%
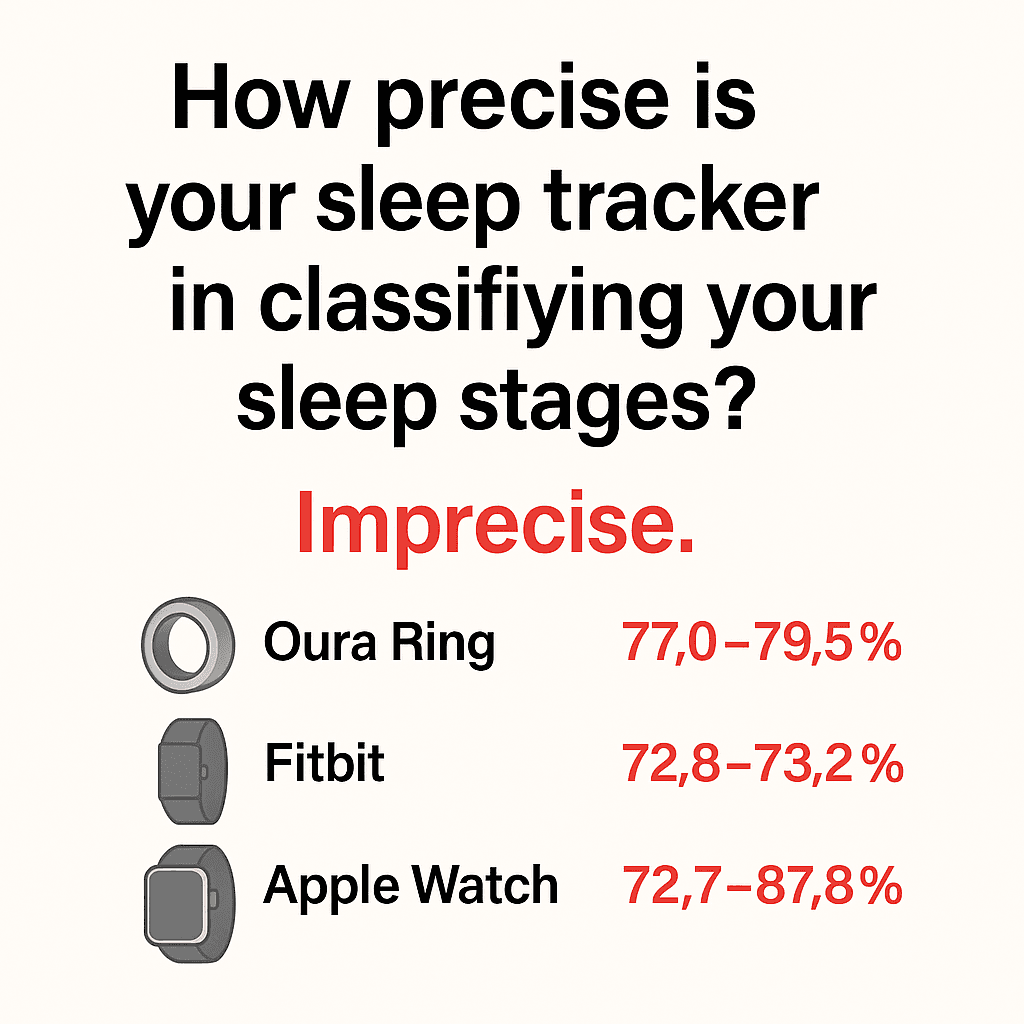
In another comparison, the Oura Ring Gen 3—often cited as one of the most advanced consumer sleep trackers—showed only about an average of 60% agreement with PSG across all sleep stages.
These devices rely on movement and heart rate variability to infer stages—but they can’t detect the brain-based signals (like spindles, arousals, or K-complexes) that define true sleep quality.
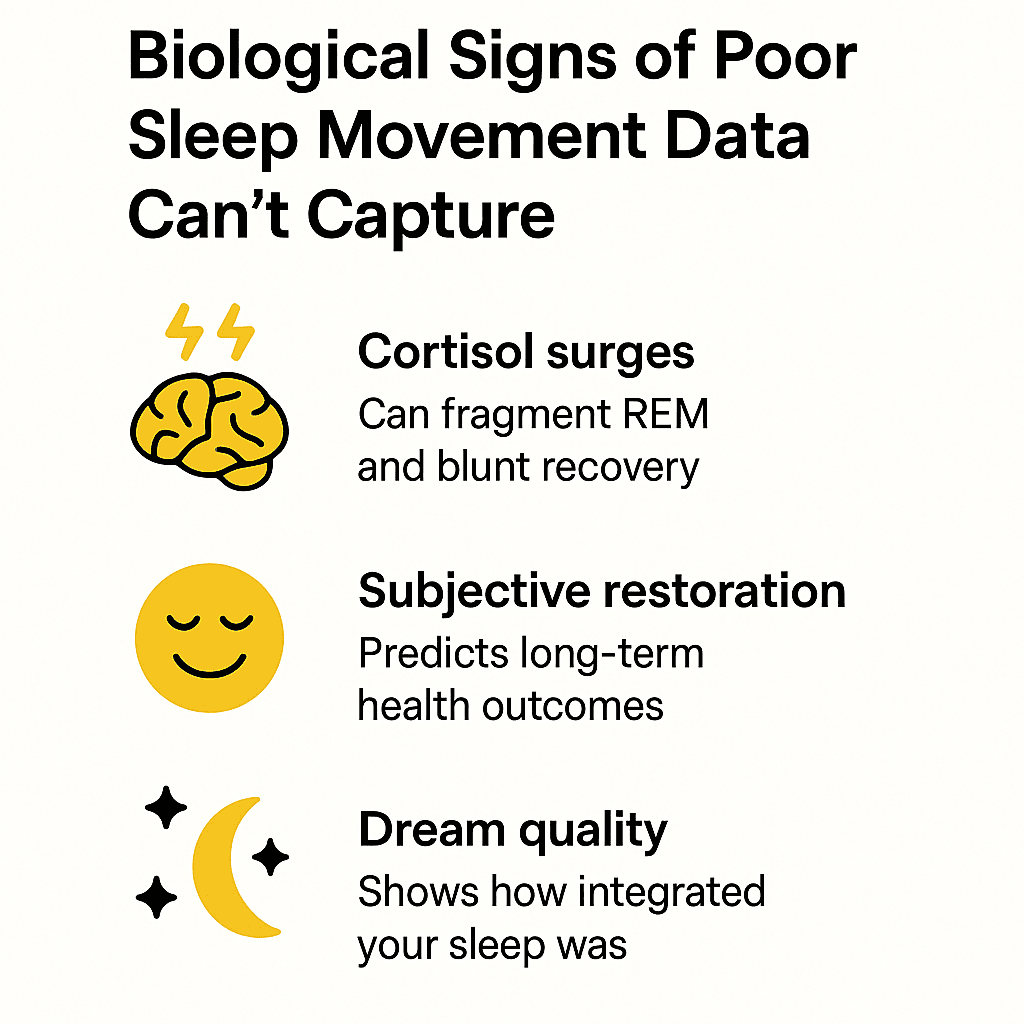
Even more important—some of the most biologically meaningful signs of poor sleep don’t show up in movement data at all.
- Low or mistimed melatonin disrupts circadian alignment, shortens total REM duration, and reduces sleep efficiency—none of which your wearable can detect directly.
- Subjective restoration—how mentally clear, emotionally stable, and physically recovered you feel—predicts long-term health outcomes independently of total sleep time.
- Dream quality, dream recall, and perceived sleep continuity reflect how integrated and complete your sleep was—but wearables can’t track any of these.
- And crucially, emotional memory consolidation—a key function of late-stage REM—is disrupted by fragmentation your ring will never register.
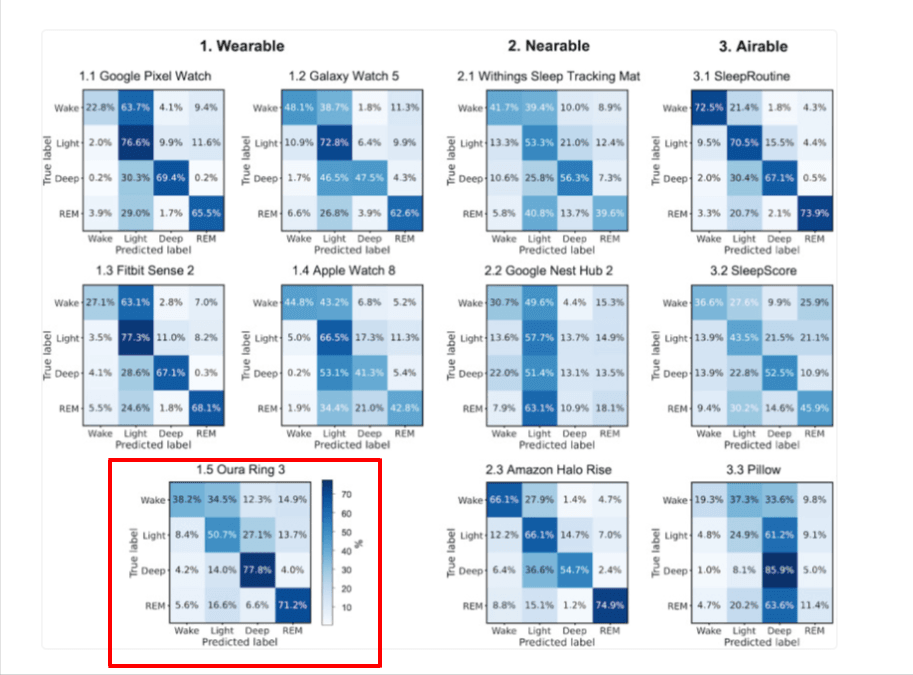
The chart below compares how accurately 11 popular consumer sleep trackers classify sleep stages—Wake, Light, Deep, and REM—against clinical-grade polysomnography (PSG). Each small grid is a confusion matrix: the rows show the actual sleep stage recorded by PSG, while the columns show what the device predicted.
- Higher percentages along the diagonal = better accuracy.
- Higher percentages off-diagonal = more misclassification.
For example, the Oura Ring Gen 3 (highlighted in red) correctly classified REM sleep 71.2% of the time and correctly classified light sleep 50.7 % of the time.
➤ What Sleep Medicine Actually Measures
In sleep science, it’s not just how long you sleep—it’s how your sleep is structured. What matters is the architecture: how stable, restorative, and well-coordinated each phase is.
These are nuances that consumer wearables simply can’t capture.
Sleep labs and EEG-based research focus on brainwave activity, not just movement or pulse. They track specific patterns that reveal the true quality of your sleep:
- Sleep spindles – brief bursts of synchronized brain activity during NREM sleep that support memory consolidation and guard against sensory disruptions.
- K-complexes – high-amplitude waveforms that act as a gatekeeper, helping maintain sleep in the face of external noise or movement.
- Microarousals – rapid (often <15 seconds) shifts in brain state that fragment sleep, even if you never become consciously awake.
You won’t remember these events—but your brain will.
Frequent sleep fragmentation disrupts synaptic repair, destabilizes mood, impairs memory consolidation, and accelerates cortical thinning & brain atrophy.
➤ The Tools Researchers Use to Detect The Sleep Fragmentation Associated with Brain Aging and Cortical Atrophy
When sleep is studied in research labs—not just tracked by a ring or wristband—clinicians go far beyond wearables. They use tools designed to detect subtle brain-level disturbances like sleep fragmentation, disrupted architecture, and even early signs of neurodegeneration.
And here’s the surprising part:
While you can’t run a full polysomnography (PSG) test at home, some of the subjective tools researchers use are actually more informative than wearables—because they capture what devices can’t.
Most wearables rely on proxies like movement and heart rate. They can’t tell you how rested you feel, whether your REM cycles were emotionally restorative, or if your brain experienced deep, uninterrupted continuity.
Validated self-assessments like the PSQI and SQS go deeper. They’ve been used for decades in sleep medicine and aging research—not to track just time in bed, but to uncover patterns of dysfunction that often go unnoticed.
And here’s why they matter even more:
Many people have never experienced truly excellent sleep—so they don’t know what they’re missing.
If you’ve adapted to years of mediocre rest, a tracking ring that says “85” may feel reassuring. But subjective tools can reveal a different story: chronic fatigue, poor emotional resilience, and subtle signs of burnout that aren’t reflected in your device’s score.
These questionnaires help you detect what your ring doesn’t: the lived quality of your sleep.
Want the step-by-step plan I use with readers 50+ to restore sleep?
PSQI: The Most Widely Used Subjective Sleep Assessment in Brain-Aging Research
The Pittsburgh Sleep Quality Index (PSQI) is not a wearable or sensor—it’s a 19-item questionnaire that measures how well you feel you’re sleeping. It’s been validated in over 900 peer-reviewed studies, translated into 56+ languages, and is widely used in both clinical and neuroscience research.
Why does this matter?
In many studies—especially those using PSG or EEG—researchers will pair objective brainwave data with tools like the PSQI to better understand the disconnect between perception and physiology.
➟ Objective architecture (measured by brainwaves)
➟ Subjective experience (captured by PSQI)
This dual approach often reveals hidden problems.
Someone may report feeling terrible despite “normal” EEG data—or vice versa, where unrecognized sleep fragmentation shows up in brainwave patterns, despite no complaints. This mismatch is known as sleep state misperception, and it’s common in aging, anxiety, depression, and insomnia.
So while PSQI is not a diagnostic or gold-standard tool, it remains a core instrument in brain health and sleep research—and it tracks changes that wearables often miss.
PSQI Self-Check: Are These Showing Up in Your Sleep?
| Domain | Signs of Dysfunction |
|---|---|
| Sleep Latency | Taking >30 minutes to fall asleep regularly |
| Sleep Duration | Averaging <6.5 hours per night |
| Sleep Efficiency | In bed 8 hrs, but sleeping <6 = <75% efficiency |
| Sleep Disturbances | Frequent waking, restlessness, light sensitivity |
| Sleep Medication Use | Growing reliance on sleep aids |
| Daytime Dysfunction | Poor focus, energy crashes, mood dips |
| Subjective Sleep Quality | “I slept, but I don’t feel recovered” |
SQS: A Broader Lens on How Sleep Feels
The Sleep Quality Scale (SQS) offers a more detailed breakdown of sleep perception. Originally developed in Korea and validated internationally, it includes 28 items across six categories:
➟ Daytime symptoms
➟ Restoration after sleep
➟ Difficulty falling or staying asleep
➟ Waking difficulty
➟ Sleep satisfaction
➟ Overall perceived quality
The SQS has been used in studies on stress, mindfulness, mood disorders, and neurodegeneration, and correlates strongly with the PSQI. In validation trials, it showed:
- ▪️ High internal consistency (α = 0.92)
- ▪️ Strong test-retest reliability (r = 0.81)
While neither PSQI nor SQS can measure brainwaves, they remain trusted tools in clinical trials, longitudinal aging studies, and sleep medicine—because they capture what devices can’t: your lived experience of sleep.
📝 Want to try them?
Download both questionnaires here:
Section 5. Still Waking Up in the Middle of the Night? The One Sleep Factor 99% of People Still Miss
Even with optimal routines—consistent bedtimes, no caffeine, morning light exposure—one major disruptor of deep, restorative sleep remains under-acknowledged: your environment.
Environmental factors can fragment sleep architecture in ways that body-worn devices rarely detect. These subtle interruptions—though often imperceptible—can impair the brain’s overnight recalibration processes. Over time, this disruption may contribute to cortical atrophy and accelerate brain shrinkage in regions essential for memory, emotional regulation, and executive control.
In other words, sleep may look sufficient by duration or movement—but still fall short of preserving the structural health of the aging brain.

➤ What Wearables Miss: Invisible Inputs That Disrupt Sleep
Several overlooked factors can interfere with deep sleep and promote sleep fragmentation:
- Indoor air quality – Elevated carbon dioxide (CO₂) or volatile organic compounds (VOCs) reduce sleep depth and respiratory stability, often without perceptible symptoms.
- Ambient noise – Low-level background sounds alter brainwave activity during slow-wave sleep, disrupting memory consolidation—even without full awakening.
- Light exposure – Artificial light at night suppresses melatonin and delays REM onset, especially from screens or poorly timed indoor lighting.
- Thermal variability – Rooms that are too warm or too cold increase micro-arousals, impairing both sleep efficiency and subjective restoration.
New clinical protocols increasingly monitor these factors in real time, recognizing their measurable impact on brain rhythms and recovery. A Johns Hopkins study found that hospital patients in rooms with natural light reported 25% higher sleep quality scores than those without—despite receiving the same care.
That’s why I monitor my sleep environment CLOSER than my nutrition and training inputs.
Subtle environmental imbalances can keep sleep fragmented, even when everything else appears “right.”
➤ The Inputs That Fragment Sleep—And How to Track Them in Real Time
The Airthings Wave Enhance is a real-time sleep environment sensor that captures the metrics wearables can’t:
CO₂, VOCs, light levels, ambient noise, temperature, and humidity.
It runs 24/7 over Wi-Fi, generates a Sleep Disruptors Report, and identifies when and why your environment broke sleep continuity—so you can address the source, not just the symptoms.
Explore the Airthings Wave Enhance here.

Sleep OS Hormones is now available as a 60-day self-guided program with dedicated systems for estrogen, progesterone, and testosterone, or bundled together for a more complete approach.
What Else Drives Brain Shrinkage (That You Might Be Overlooking)?
Brain atrophy isn’t just a symptom of old age—it’s a structural response to years of disrupted recovery.
Fragmented sleep, REM loss, and poor circadian alignment all contribute. But they’re not the only causes.
If you’re curious about other underrecognized causes of brain aging—especially in individuals with “normal” labs—read these next:
➤ Vitamin B12 and Brain Fog: What Blood Tests Miss About Early Cognitive Decline →
➤ Vitamin D and Cognitive Aging: 5 Steps to Protect Your Brain →
Are you still taking melatonin—and still waking up tired?
You’re not alone. Most people use melatonin to fix fragmented sleep, but it rarely delivers on that promise. Here’s why it often fails—and what to do instead:
➤ Melatonin for Sleep? Why It Often Fails—and What to Do Instead →
Frequently Asked Questions (FAQ)
What is brain atrophy?
Brain atrophy refers to the loss of neurons and the connections between them, leading to shrinkage in brain volume.
This process is normal with aging but can be accelerated by poor sleep, neurodegeneration, and inflammation. Brain shrinkage on MRI often reveals this loss in regions like the cortex and hippocampus—areas linked to memory, planning, and emotional regulation. Cortical atrophy is one of the most common visual signs of brain aging and is measurable even in high-functioning adults in their 40s.
What causes brain atrophy?
Brain atrophy can be caused by aging, neurodegenerative diseases, and lifestyle factors like chronic sleep disruption.
One of the lesser-known brain shrinkage causes is sleep fragmentation—brief, repeated arousals that interrupt sleep stages like REM and slow-wave sleep. Studies show that even without sleep deprivation, poor sleep architecture can trigger cortical atrophy, especially in the frontal lobes and default mode network regions tied to memory and executive function.
Can lack of sleep cause brain atrophy?
Yes, research shows that poor sleep quality—including sleep fragmentation—can accelerate structural brain changes.
MRI studies link chronic midlife sleep disturbances to greater brain age gaps and cortical thinning. This means that even if total sleep time is adequate, fragmented or shallow sleep may still cause the brain to shrink faster, particularly in Alzheimer’s-vulnerable areas like the precuneus and inferior parietal lobule.
What is sleep fragmentation?
Sleep fragmentation is when your sleep is repeatedly interrupted by micro-awakenings, even if you don’t fully wake up.
These short disruptions pull the brain out of deep sleep stages and prevent full restoration. Over time, sleep fragmentation increases your risk of brain shrinkage by interfering with glymphatic waste clearance, synaptic pruning, and emotional memory consolidation—all critical for long-term brain health and neuroprotection. That’s why I use an environmental sleep monitor that tracks CO₂, noise, and other inputs most wearables miss.
What are the effects of fragmented sleep?
Fragmented sleep leads to poorer emotional regulation, impaired memory, and faster brain shrinkage.
One study found that higher sleep fragmentation predicted cortical atrophy in the orbitofrontal cortex—an area involved in decision-making and self-control. Even without cognitive symptoms, this structural decline shows up on MRI as thinning in key regions, suggesting that sleep fragmentation may be an early contributor to brain aging.
How to reverse brain shrinkage?
While full reversal of brain shrinkage is unlikely, certain interventions may help preserve or restore brain volume over time.
Improving sleep quality—especially by reducing sleep fragmentation—can slow further atrophy. Some studies even show modest increases in gray matter volume, particularly in areas like the hippocampus and prefrontal cortex, through aerobic exercise, mindfulness, and sleep restoration.
REM sleep protection, circadian alignment, and eliminating environmental disruptors like CO₂ and ambient noise may help support neuroplasticity. MRI-visible changes may not fully reverse, but functional improvements are possible with consistent lifestyle upgrades.
References
- Lee T, Cho Y, Cha KS, Jung J, Cho J, Kim H, Kim D, Hong J, Lee D, Keum M, Kushida CA, Yoon IY, Kim JW. Accuracy of 11 Wearable, Nearable, and Airable Consumer Sleep Trackers: Prospective Multicenter Validation Study. JMIR Mhealth Uhealth. 2023 Nov 2;11:e50983. doi: 10.2196/50983. PMID: 37917155; PMCID: PMC10654909.
- Robbins, R.; Weaver, M.D.; Sullivan, J.P.; Quan, S.F.; Gilmore, K.; Shaw, S.; Benz, A.; Qadri, S.; Barger, L.K.; Czeisler, C.A.; et al. Accuracy of Three Commercial Wearable Devices for Sleep Tracking in Healthy Adults. Sensors 2024, 24, 6532. https://doi.org/10.3390/s24206532
- Cavaillès C, Dintica C, Habes M, Leng Y, Carnethon MR, Yaffe K. Association of Self-Reported Sleep Characteristics With Neuroimaging Markers of Brain Aging Years Later in Middle-Aged Adults. Neurology. 2024 Nov 26;103(10):e209988. doi: 10.1212/WNL.0000000000209988. Epub 2024 Oct 23. PMID: 39442064; PMCID: PMC11498938.
- Kokošová V, Filip P, Kec D, Baláž M. Bidirectional Association Between Sleep and Brain Atrophy in Aging. Front Aging Neurosci. 2021 Dec 8;13:726662. doi: 10.3389/fnagi.2021.726662. PMID: 34955805; PMCID: PMC8693777.
- Vidal-Pineiro, D., Parker, N., Shin, J. et al. Cellular correlates of cortical thinning throughout the lifespan. Sci Rep 10, 21803 (2020). https://doi.org/10.1038/s41598-020-78471-3
- Deantoni, M., Reyt, M., Dourte, M. et al. Circadian rapid eye movement sleep expression is associated with brain microstructural integrity in older adults. Commun Biol 7, 758 (2024). https://doi.org/10.1038/s42003-024-06415-y
- Cho G, Mecca AP, Buxton OM, Liu X, Miner B. Lower slow wave sleep and rapid eye-movement sleep are associated with brain atrophy of AD-vulnerable regions. bioRxiv [Preprint]. 2025 Jan 14:2025.01.12.632386. doi: 10.1101/2025.01.12.632386. Update in: J Clin Sleep Med. 2025 Mar 31. doi: 10.5664/jcsm.11630. PMID: 39868141; PMCID: PMC11761512.
- Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, Fjell AM. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014 Sep 9;83(11):967-73. doi: 10.1212/WNL.0000000000000774. Epub 2014 Sep 3. PMID: 25186857; PMCID: PMC4162301.
- Lim AS, Fleischman DA, Dawe RJ, Yu L, Arfanakis K, Buchman AS, Bennett DA. Regional Neocortical Gray Matter Structure and Sleep Fragmentation in Older Adults. Sleep. 2016 Jan 1;39(1):227-35. doi: 10.5665/sleep.5354. PMID: 26350471; PMCID: PMC4678338.
- A. Shahid et al. (eds.), STOP, THAT and One Hundred Other Sleep Scales